Background
The term CTO describes a chronic total coronary occlusion of at least three months duration with absolutely no flow through the lesion itself (TIMI 0 flow).
The basic patho-histological features of a CTO comprise the proximal fibrotic or calcified cap of the occlusion, followed by a segment of loose fibrous tissue or organised thrombus with various degrees of adventitial and intraluminal neovascularisation, and the distal cap, which is often tapered.
Indications
1) To relieve exercise limiting symptoms of angina or dyspnoea and, in less symptomatic patients, to resolve ischaemia as detected by non-invasive stress testing.
2) To improve regional LV dysfunction in the territory of the occluded artery provided that there is residual viability as assessed by magnetic resonance imaging with late contrast enhancement.
3) To improve the prognosis of a patient as there is considerable risk of future progression of coronary artery disease in the remaining patent arteries.
An indispensable prerequisite for opening a CTO is the presence of collaterals to supply the distal occluded segment. In the absence of collaterals, no viable myocardium would have survived.
The interventional techniques used to open such a CTO have advanced considerably over the past decade, with the retrograde approach as an additional option. Still, the primary approach remains the antegrade technique which in most cases utilises new wires and tools.
Methods
Optimal angiographic visualisation of the vessel distal to the occluded segment is the prerequisite for a successful procedure. This requires, in most cases, a second sheath for injection into the contralateral collateral donor artery. The next initial step is to ensure optimal guide catheter back-up, not only for the wire passage, but also for balloon and stent advancement. The use of a microcatheter as support is recommended as it facilitates the wire manipulation greatly, but over-the-wire balloons can be used as well.
Guidewire selection incorporates a great deal of personal preferences and experience. The wire selection depends on the planned approach to the occlusion. The three classical approaches are: the drilling technique, the penetrating technique and the sliding technique. Recently, with the availability of new soft tapered wires, an initial option has been to probe for microchannels, which, however, do not typically traverse the lesion, but may provide entry into the occlusion, after which the wire may proceed within loose tissue, even in calcified lesions. This latter approach as a minimal traumatic initial step is favoured by many operators. Each of these approaches may be selected according to lesion morphology from the onset, but often needs to be changed during the procedure itself (Figure 1). Flexibility and adaptation is required throughout the entire procedure (Figure 2).
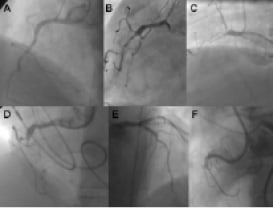
Figure 1. Examples of angiographic occlusion morphology. Cases A-C with tapered entry: A was successfully crossed with a Fielder XT (Abbott Vascular, Redwood City, CA, USA) as the initial choice; B was tried with Fielder XT, but was not crossed to the distal lumen, and wire step-up was necessary (Miracle 3G, Confianza Pro 9; ASAHI Intecc, Aichi, Japan); C Fielder XT and Miracle 3G could not penetrate, but Confianza Pro 9 did. Cases D-F with blunt entry or side branch take-off: D penetration was achieved with Progress 200T (Abbott Vascular, Redwood City, CA, USA) after Fielder XT failed; E penetration was achieved with Confianza Pro 9 after Fielder XT failed; F was penetrated by Confianza Pro 9 initially but went subintimal;< this case was solved after switching to the retrograde approach.
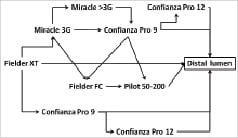
Figure 2. Possible iterations of the wire selection for an antegrade approach to CTOs reflecting the authors preferences at this time. Due to wire development, changes need to be considered. One major change in the strategy was the upfront use of the Fielder XT in the majority of cases. Typical wires are named, but they can be replaced by wires from other vendors if the operator is experienced and familiar with these wires. Basically switches from soft PTFE wires (Fielder and Pilot) to hard wires (Miracle 3G, Confianza Pro) and back should be considered during the process.
When the true lumen wire passage fails, the first wire is then used as a guide for a second parallel wire slightly deviating from the initial course to successfully enter the distal lumen (Figure 3). In case of persistent subintimal passage, several methods may be applied for a re-entry including new device technologies.
After the guidewire is successfully advanced across the occlusion, and most importantly, its correct intravascular position is checked in at least two orthogonal views with a contralateral injection, a modern low profile balloon is advanced, followed by larger balloons. There is a clear indication to use drug-eluting stents in CTOs to ensure a high long-term patency.
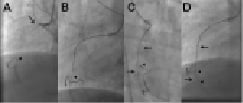
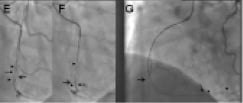
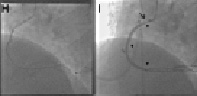
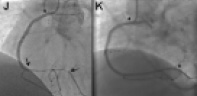
Figure 3. Example of the parallel wire technique. A. An approximate 35 mm long occlusion of the proximal right coronary artery (arrow), with visualisation of the distal target (arrow head) through a contralateral injection. The occlusion has a short tapered segment. Due to the proximal occlusion site, a less aggressive JR 4 side hole guide is selected (Launcher, Medtronic), using a 7 Fr for increased support. B. After failed advancement of a Fielder XT (ASAHI Intecc), a Miracle 3G (ASAHI Intecc) is advanced close to the distal target with a side branch take-off as an unfavourable characteristic. C. With no further advancement, the supporting Finecross microcatheter (Terumo Corp., Tokyo, Japan) is advanced into the occluded segment (full arrow), and the wire exchanged for a Confianza Pro 9 (ASAHI Intecc), which passed the distal target (arrow head), but went subintimal (arrow). D. The same situation in the lateral view: imaging from orthogonal planes is essential for the further guidewire manipulation. E. With a subintimal wire position, a second wire, again a Confianza Pro is advanced with support of a Finecross catheter (which was removed from the first wire) parallel to the first wire (arrow), with the intention to deviate distally from the false course towards the true lumen (arrow heads). Initially the second wire is posterior to the first wire, but subintimal (full arrow). F. With slight manipulation, the wire is redirected into the true lumen entering at the side branch take-off. Contrast filling around the tip of the wire (full arrow). G. Confirmed and advanced in the lateral view distally into a posterolateral branch (full arrow). The first wire (arrow) is left in place to anchor the system for lesion crossing with microcatheter or balloon. Then the Finecross could be advanced beyond the occlusion, and an extra support floppy wire (Abbott Vascular) exchanged for the stiff recanalisation wire. Figure 3b. H: After balloon dilatation starting with a 1.5 mm balloon Sprinter Legend (Medtronic, Minneapolis, MN, USA). The diffuse lumen narrowing of the actually dominant right coronary artery is caused by the slow re-establishing of intraluminal pressure. It is difficult to discern vasoconstriction from obstructing plaque. Repeated injection of nitroglycerine may help, as well as optional intravascular ultrasound to decide this issue. I. After further balloon dilatation with a 2.5×30 mm Maverick (Boston Scientific) two Xience Prime stents (Abbott Vascular) (3.5×38 mm) were implanted starting from the crux cordis close to the ostium (between arrow heads). At the ostium a residual lesion (arrow) is not covered, as well as diffuse narrowing in the long proximal part of the posterolateral system. J. An additional Xience stent (3.5×8 mm) is placed in the ostium (arrow head), and additional stent will be required distally (between arrows). K. With two additional Xience stents (2.75×38 mm distal, 3.0×18 mm proximal) this dominant right coronary artery system is reconstructed between the five stents, most importantly with all side branches open. A so called full metal jacket was not avoidable in this case.
Complications
Published data show no difference in complication rates between occlusive and non-occlusive lesions, even with advanced techniques. Pericardial tamponade is the feared acute complication, which occurs rarely in case of wire perforations, and can be most often avoided by careful control of the wire tip position. The operator needs to be experienced in placing pericardial drainage, and be familiar with techniques to seal the source of the pericardial effusion.
Complications from contrast volume and radiation exposure are related to extended procedure times.

