
It is 2050. A patient presents with exertional angina and a right coronary artery chronic total occlusion (CTO). Preprocedural modelling reveals a 39% increase in exercise capacity and 95% complete symptom resolution upon recanalisation. Multimodality scanning shows a clear proximal cap, a length of 35.4 mm with 56% eccentric medial calcification, a negatively remodelled distal vessel with a mean diameter of 2.45 mm, with potential for expansion by 0.32 mm post recanalisation and collateral circulation by septals with three potentially appropriate channels for retrograde crossing with an 88.9% likelihood of successful guidewire and 88.5% likelihood of microcatheter crossing. Upon three-dimensional mapping of the optimal crossing routes, the self-propelled CTO recanaliser is inserted through a 0.1 Fr entry point, successfully crosses the occlusion within one minute and delivers an optimally composed scaffold that restores flow with 0.001% likelihood of acute or chronic failure without need for additional medications. The patient is back to work 20 minutes later and resumes exercise later in the day, experiencing an improvement in symptoms exactly as predicted.
Back to 2020. The same patient undergoes a failed CTO percutaneous coronary intervention (PCI) attempt at a moderately experienced centre. Two months later he is referred to a more experienced centre. Coronary computed tomography angiography is performed to clarify proximal cap ambiguity. Antegrade and retrograde CTO recanalisation is attempted using femoral and radial access. Three hours later, and after multiple changes in procedural strategy, the lesion is successfully crossed, and three drug-eluting stents are placed under intravascular ultrasound guidance. He is dismissed the following day with a recommendation for >1-year antiplatelet therapy and a gradual return to daily activities over the next five days. Over the following year he is facing a 5-10% risk of in-stent restenosis and 1-2% risk of stent thrombosis1.
Clearly, the 2020 techniques and outcomes for recanalising coronary CTOs lag behind what will probably be possible in the future. Can today’s outcomes be improved? Absolutely yes! Seven CTO PCI best practices have emerged for achieving high success and minimising the risk of subsequent complications2.
The first best practice is careful angiographic (and clinical) review, which is often summarised in CTO scores, i.e., numerical scales that provide a summary estimate of the difficulty of recanalisation and the likelihood of success. The initially described and most widely used is the J-CTO score3, followed by several other scores, such as the Prospective Global Registry for the Study of Chronic Total Occlusion Intervention (PROGRESS CTO)4, and the CABG, Age, Stump anatomy, Tortuosity, Length of CTO, and Extent of calcification (CASTLE)5 scores (Figure 1). In this issue of EuroIntervention, Kalogeropoulos et al compared the J-CTO and CASTLE scores in terms of predicting CTO PCI technical success at a large, experienced centre, showing overall similarly poor performance (area under the curve 0.698 for J-CTO vs 0.676 for CASTLE)6.
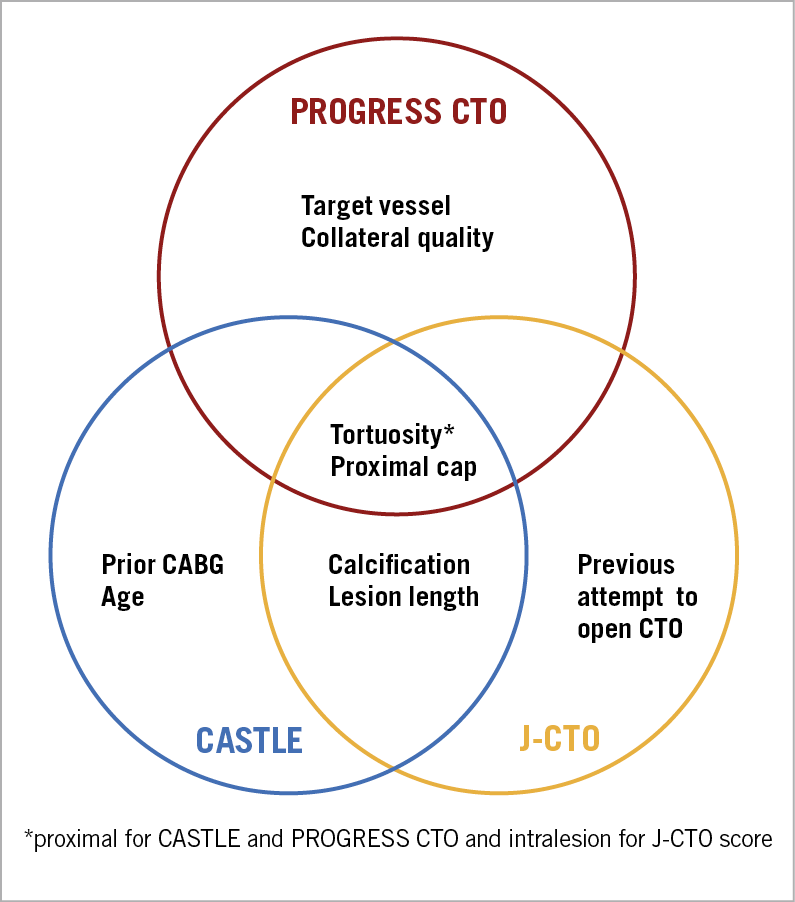
Figure 1. Overlap in the parameters included in three chronic total occlusion percutaneous coronary intervention scores. CABG: coronary artery bypass graft surgery; CTO: chronic total occlusion
Although some operators remain sceptical about the use of CTO PCI scores, we believe that CTO scores should be calculated in every case for several reasons:
1. Their calculation necessitates careful review of the angiogram prior to CTO PCI, which leads to better understanding of the target lesion, better crossing strategy selection, a higher likelihood of success and a lower risk of complications.
2. They facilitate discussion with the patients and their families about what to expect.
3. They facilitate workflow planning, case scheduling and the resources potentially required.
4. They facilitate operator and patient matching: highly complex cases should be performed by highly experienced operators, whereas less complex cases could be performed by less experienced operators. CTO PCI scores can be especially useful for interventional cardiologists early in their learning curve.
Which score is better? Should CASTLE be the preferred score going forward? Ideally, multiple scores should be calculated for the following reasons:
1. Each score has a different endpoint. While most scores use technical success as the endpoint, J-CTO uses guidewire crossing within 30 minutes, although it also correlates with procedural success.
2. Each score was developed in different patient populations and operators. The CASTLE score is based on European operators and incorporates prior CABG, which varies significantly between continents.
3. Each score was calculated at a different time period. For example, the J-CTO score was developed from 494 CTO PCIs performed in Japan between 2006 and 2007, whereas the CASTLE score was derived from 20,000 cases included in the EuroCTO registry between 2008 and 2014. Since CTO PCI is continuously evolving, newer scores might be more accurate in predicting success.
4. Although there is significant overlap, each score includes different variables (Figure 1).
5. Some variables can be challenging to interpret. For example, the J-CTO score includes prior failed attempt, which could be due to high lesion/patient complexity or attempt by an inexperienced operator.
CTO PCI is constantly evolving and what is performed today may (and probably will) become outdated in the near future. Until then, CTO PCI score calculation and the associated comprehensive angiographic review remain critical for both maximising success and, most importantly, optimising the safety of the procedure.
Conflict of interest statement
E.S. Brilakis reports receiving consulting/speaker honoraria from Abbott Vascular, American Heart Association (associate editor Circulation), Biotronik, Boston Scientific, Cardiovascular Innovations Foundation (Board of Directors), CSI, Elsevier, GE Healthcare, Infraredx, Medtronic, Siemens, and Teleflex, research support from Regeneron and Siemens, and being a shareholder in MHI Ventures. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

