
Abstract
Aims: The aim of this study was to compare percutaneous coronary intervention (PCI) outcomes in relation to stent optimisation profiles between in-stent chronic total occlusions (CTOs) and de novo CTOs.
Methods and results: We evaluated 1,516 consecutive patients who underwent PCI for 147 in-stent CTOs (9.3%) and 1,439 de novo CTOs between 2007 and 2018. The primary endpoint was target vessel failure (TVF) consisting of a composite of cardiac death, target vessel-related myocardial infarction, or target vessel revascularisation. The final post-stenting intravascular ultrasound (IVUS) images were analysed. Target lesion complexity reflected by the Japanese CTO score was similar, albeit calcification was more prevalent in de novo CTOs, whereas occlusion length >20 mm was more frequent in in-stent CTOs. The technical success (88.4% vs 87.5%, p=0.84) and in-hospital adverse event (1.4% vs 3.6%, p=0.26) rates were similar between CTO types. Among those who received drug-eluting stents, the five-year TVF (11.0% vs 10.7%, p=0.99) and target vessel revascularisation (4.2% vs 3.7%, p=0.81) rates were similar between groups. Total stent length, minimum stent area (5.4±1.8 vs 5.5±1.8 mm2, p=0.77), and maximal plaque burden of the reference segments were largely comparable between groups.
Conclusions: In-stent CTO PCI with drug-eluting stents optimised by IVUS guidance offers as acceptable long-term clinical results as those achieved in de novo CTOs.
Introduction
Percutaneous coronary intervention (PCI) has become a preferred revascularisation strategy for patients with coronary artery disease, providing the benefit of effective relief for anginal symptoms and resolution of objective evidence of ischaemia1. However, although the incremental yearly rate of in-stent restenosis has become very low recently, it continues to occur over time, mitigating the advantages achieved by stent-based treatments. Although non-occlusive in-stent restenosis is readily treated by repeat PCI, its use for in-stent chronic total occlusions (CTOs) is technically challenging and was traditionally associated with suboptimal procedural success rates2,3,4. Accordingly, in-stent CTOs are often treated medically or surgically based on the disease extent of other coronary arteries.
With the various available devices and strategies, PCI has become more liberally used to treat in-stent CTOs, with the procedural success rate improving results as seen in more recent registries. Nevertheless, controversy persists about the efficacy of PCI for in-stent CTOs, mainly due to the perception of inferior treatment durability compared to that seen for de novo stenotic or CTO lesions5,6. Therefore, we evaluated the procedural and long-term outcomes of in-stent CTO PCI and compared them with those of contemporary de novo CTO PCI with specific focus on post-procedural stent optimisation.
Methods
STUDY POPULATION
The study cohort included patients prospectively enrolled in the CTO registry of the Asan Medical Center, Seoul, South Korea, between January 2007 and June 2018. This registry enrolled patients who had angina or evidence of ischaemia and had a target CTO located in a major epicardial vessel with a reference vessel diameter ≥2.5 mm. Clinical, procedural, and outcome data were recorded in dedicated databases by independent research personnel. Telephone interviews and medical records of other hospitals were also obtained to ensure an accurate assessment of clinical endpoints as necessary. Coronary angiograms performed at baseline and after stenting were reviewed by independent angiographers in the angiographic core laboratory. CTO lesion complexity was assessed by calculating the Japanese CTO (J-CTO) score for each case. In each procedure, the use of specialised devices and techniques and the choice of drug-eluting stent (DES) type were left to the operator’s discretion. Stent implantation was performed under intravascular ultrasonography (IVUS) guidance unless technically infeasible7. The usual manner of IVUS-guided PCI in our medical institution is as follows: 1) selection of the optimal landing zone and stent length to cover the residual disease adjacent to the lesion; 2) selection of an appropriate stent size considering the external elastic lamina diameters of the distal reference as well as the ability of the particular stent platform to accommodate expansion to the proximal reference diameter; and 3) maximisation of the final stent area using a non-compliant balloon (ideally to achieve a minimal stent area of ≥5.5 mm2 based on our previous report)8. The local institutional review board approved this study and all patients provided written informed consent.
DEFINITIONS AND ENDPOINTS
The definition of CTO used for inclusion in the registry has been published elsewhere9. The database was reviewed to identify patients who underwent PCI for in-stent CTO, which was defined as a CTO lesion located within a previously implanted stent or involving the 5 mm segment proximal or distal to the stent edge.
Technical success was defined as the restoration of Thrombolysis In Myocardial Infarction (TIMI) flow grade 3 with residual stenosis <30% in the target lesions. In-hospital serious adverse events included any of the following before hospital discharge: death, myocardial infarction (MI), urgent target vessel revascularisation (TVR) with PCI or bypass surgery, cardiac tamponade requiring intervention, and stroke.
The primary endpoint of interest was target vessel failure (TVF), defined as a composite of cardiac death, target vessel-related MI, or TVR during follow-up after a successful procedure. Death was considered cardiac unless an unequivocal, non-cardiac cause was established. MI included procedure-related or spontaneous MI. Procedure-related MI was indicated by peak creatine kinase-myocardial band >10 times the upper reference limit within 48 hours post procedure, while spontaneous MI was defined as any creatine kinase-myocardial band or troponin increase above the upper limit of normal with ischaemic symptoms or signs during follow-up. Target vessel-related MI was defined as MI attributed to the vessel in which the CTO PCI was performed. TVR was defined as any repeat revascularisation using PCI or bypass surgery of the index CTO vessel. For all clinical endpoints, only the first procedure for each patient was considered in the analyses.
INTRAVASCULAR ULTRASOUND MEASUREMENTS
IVUS imaging was performed using motorised transducer pullback (0.5 mm/sec) and a commercial scanner (Boston Scientific Scimed, Minneapolis, MN, USA) consisting of a rotating 30- or 40-MHz transducer within a 3.2 Fr imaging sheath. The analysis of final post-stenting IVUS images was performed by independent personnel who were blinded to the patients’ baseline characteristics and clinical outcomes. Computed planimetry (echoPlaque™ 3.0; Indec Systems, Santa Clara, CA, USA) was used to measure the minimal stent area and the lumen or external elastic membrane area of the proximal and distal reference segments and to identify the presence of dissection or haematoma at the stent edge or stent malapposition.
STATISTICAL ANALYSIS
Continuous variables are presented as mean±standard deviation, while categorical variables are shown as number (percentage). Continuous variables were compared using the Student’s t-test or the Wilcoxon rank-sum test, while categorical variables were compared using the χ2 or Fisher’s exact test as appropriate. Cumulative event rates and probability curves were generated using the Kaplan-Meier method. Follow-up was censored at the date of the last follow-up or at five years, whichever came first. Cox proportional hazards models were used to estimate the risk of adverse events of the in-stent CTO group compared with that in the de novo CTO group. Risk-adjusting variables included age, sex, body mass index, hypertension, diabetes, chronic kidney disease, prior stroke, peripheral vascular disease, left ventricle ejection fraction, presence of left main disease, presence of multivessel disease, and J-CTO score. The final models for each endpoint were determined using backward stepwise elimination procedures in which the least significant variable was removed one at a time from the full model.
Propensity score-matching analysis was further performed to control for potential confounders and minimise any selection biases. The propensity score was estimated non-parametrically by fitting a logistic regression model using variables outlined in Supplementary Table 1. Matching was performed with the use of a 1:1 matching protocol with a calliper width equal to 0.2 of the standard deviation of the logit of the propensity score. Standardised differences were estimated for all the baseline covariates before and after matching; values of less than 10% for a given covariate indicate a relatively small imbalance. A Cox proportional hazards regression model with robust standard errors that accounts for the clustering of the pairs was used to compare the risks of outcomes in the matched cohort. The p-values were two-sided, and those <0.05 were considered significant. Data analyses were performed using R software version 3.5.3 (R Foundation for Statistical Computing, Vienna, Austria).
Results
INITIAL PROCEDURAL RESULTS
A total of 1,586 CTO PCI procedures were attempted in 1,516 patients during the study period. Among them, 147 (9.3%) procedures targeted in-stent CTOs. Seven (4.8%) in-stent CTO procedures were at least a second attempt for the index CTO lesion. The target lesion complexity reflected by the J-CTO score was similar between the in-stent and de novo CTO groups (2.0±1.1 and 1.9±1.1, respectively; p=0.32). The technical success rate for in-stent CTOs was similar to that for de novo CTOs (88.4% vs 87.5%; p=0.84). During the index hospitalisation, serious adverse events occurred at similar rates (1.4% vs 3.6%; p=0.26) between the two groups (Table 1).
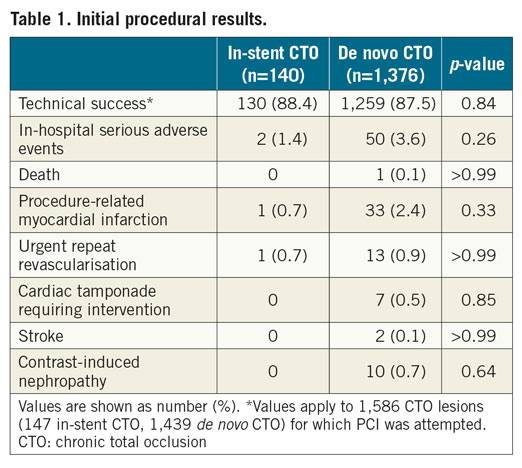
PATIENT AND PROCEDURAL CHARACTERISTICS
A total of 1,257 patients successfully underwent DES implantation for 1,278 CTO lesions and were included in the outcome analysis (Figure 1). Among the 93 patients with in-stent CTO, the type of stent leading to in-stent CTO was a bare metal stent in 30 (32%) and a drug-eluting stent in 63 (68%). The cohort comprised 1,060 (84.3%) men; the mean patient age was 60.6 years. Overall, there was no significant difference in patient demographic or clinical characteristics between the in-stent and de novo CTO groups except that the former included more patients with prior MI, PCI, and bypass surgery (Table 2). Baseline angiographic and procedural data are listed in Table 3. Although there were no differences in target CTO vessel, J-CTO score, or degree of collateralisation, calcification within the CTO was more prevalent in the de novo CTO group, while occlusion length >20 mm occurred more frequently in the in-stent CTO group. The primary antegrade approach was more commonly used for in-stent CTOs than for de novo CTOs (93.8% vs 86.7%; p=0.07). Accordingly, the final wire passage was successful with more frequent use of medium or high penetration force wires into in-stent CTOs than in de novo CTOs (48.4% vs 35.1%; p=0.01). No cases of in-stent CTO required subintimal crushing of the previous stent.
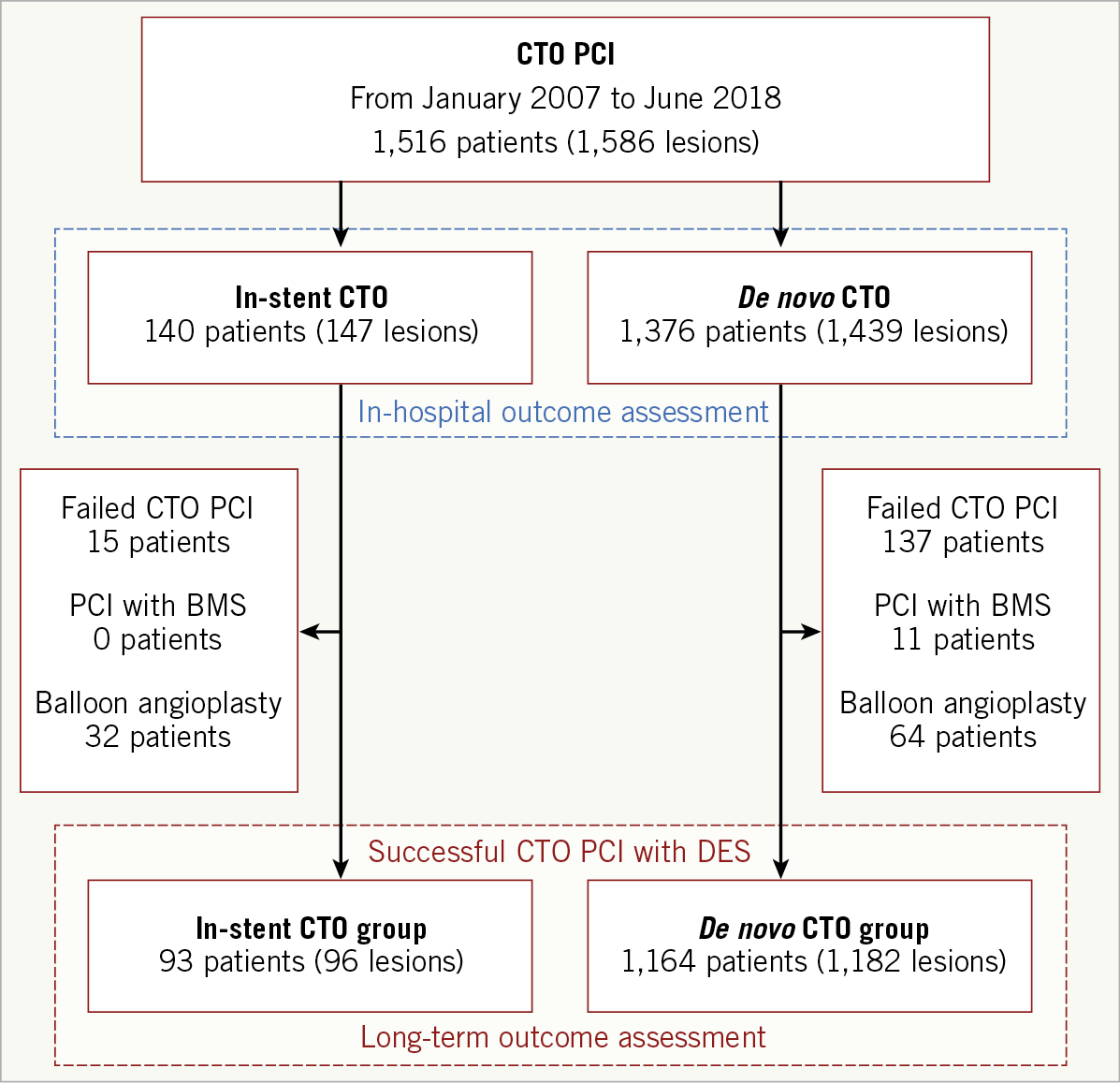
Figure 1. Study flow chart.
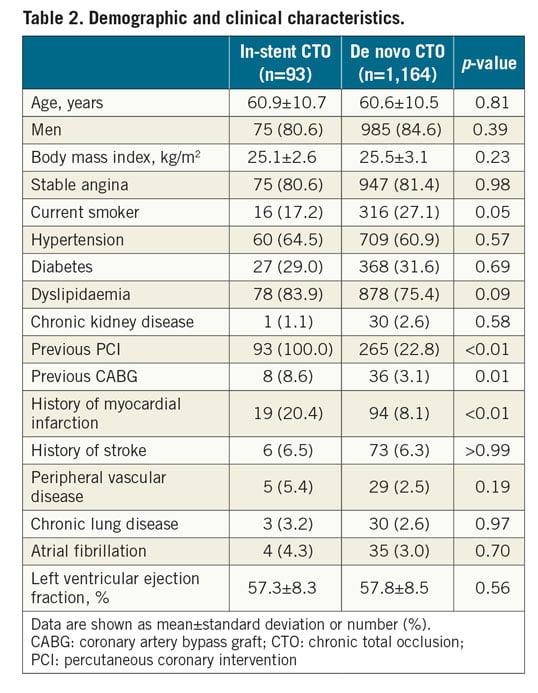
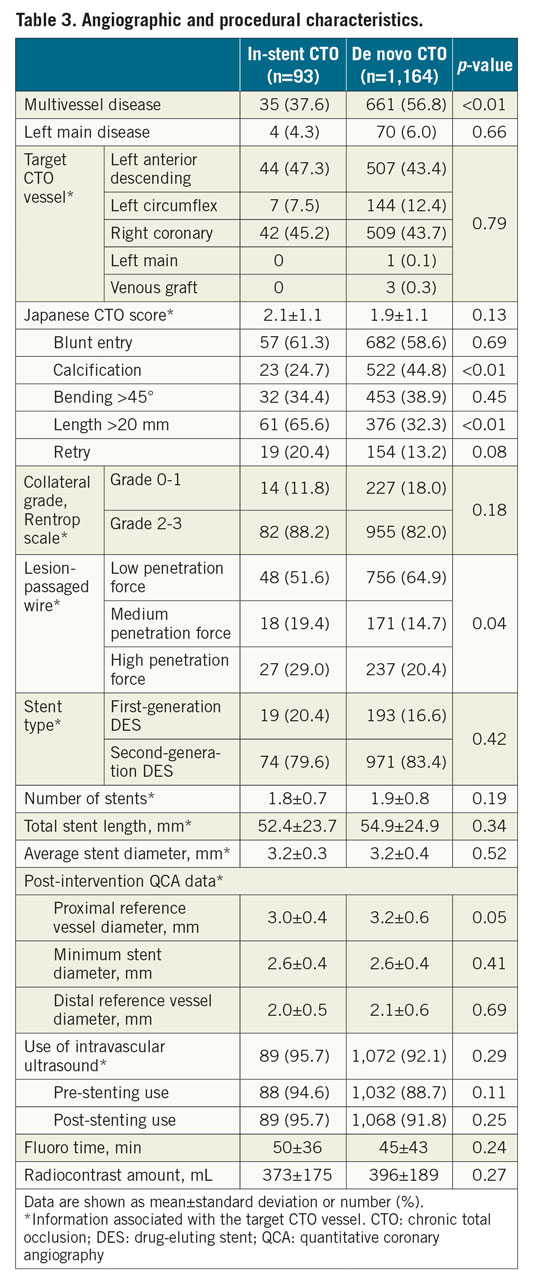
STENT OPTIMISATION
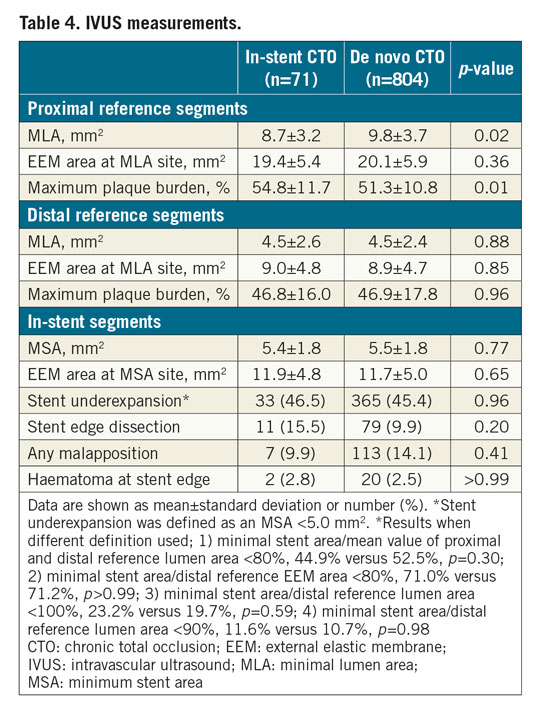
There was no significant intergroup difference in mean length (52.4±23.7 mm with in-stent CTO, 54.9±24.9 mm with de novo CTO; p=0.34) and nominal diameter (3.2±0.3 mm vs 3.2±0.4 mm; p=0.52) of the implanted stents at the target CTO lesion. IVUS was used for stent optimisation in more than 90% of the study cohort. Among them, full images of the pre- and the final post-stenting IVUS evaluations at the target vessel were available in 71 and 804 patients in the in-stent and de novo CTO groups, respectively (Table 4). Among the in-stent CTOs, underexpansion of the initial stent (defined as an MSA <5.0 mm2) was detected in 16 cases (22.5%). Although the absolute differences were small, minimum lumen area (difference of 1.1 mm2) and maximal plaque burden (difference of 3.5%) at the proximal reference segment were in favour of the de novo CTO group. However, other important IVUS parameters such as the minimum stent area (5.4±1.8 vs 5.5±1.8 mm2; p=0.77) and the maximal plaque burden of the distal reference segment (46.8% vs 46.9%; p=0.96) were similar between the two groups. Accordingly, quantitative coronary angiographic measurement showed similar minimum stent diameter between the groups. Also, there were no significant intergroup differences in the frequency of stent underexpansion, stent malapposition, or any degree of stent edge dissection or haematoma.
CLINICAL OUTCOMES
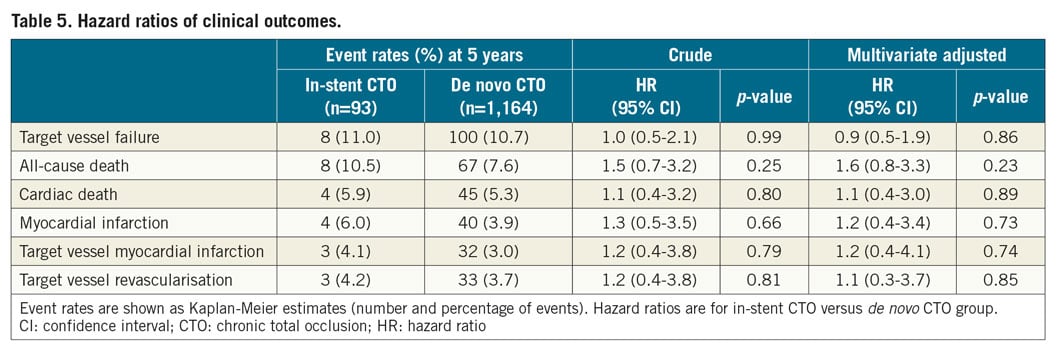
The median follow-up period for the successful CTO PCI cohort was 5.2 years (interquartile range, 2.1-8.3 years). During the follow-up period, a total of 75 patients died, 49 of cardiac causes. MI occurred in 44 patients; TVR was performed in 36 of them. The Kaplan-Meier curves for the clinical endpoints are shown in Figure 2. The cumulative rates of TVF (11.0% vs 10.7%; p=0.99) and TVR (4.2% vs 3.7%; p=0.81) were similar between the in-stent and de novo CTO groups. The cumulative rates of death and target vessel MI were comparable between the two groups. During follow-up, there were 9 cases (2 in the in-stent CTO and 7 in the de novo CTO group) of definite stent thrombosis without a significant between-group difference (p=0.10). In the adjusted analysis using a multivariate Cox regression model, the risk of TVF remained comparable between the two groups, as did that for the other secondary study endpoints (Table 5). After propensity score matching, there were 88 matched pairs of patients. The risks of TVF (hazard ratio [HR] 0.9, 95% confidence interval [CI]: 0.3-2.4, p=0.77), as well as the individual outcomes, were in line with the results of the multivariable analysis (Supplementary Table 1, Supplementary Table 2, Supplementary Figure 1).
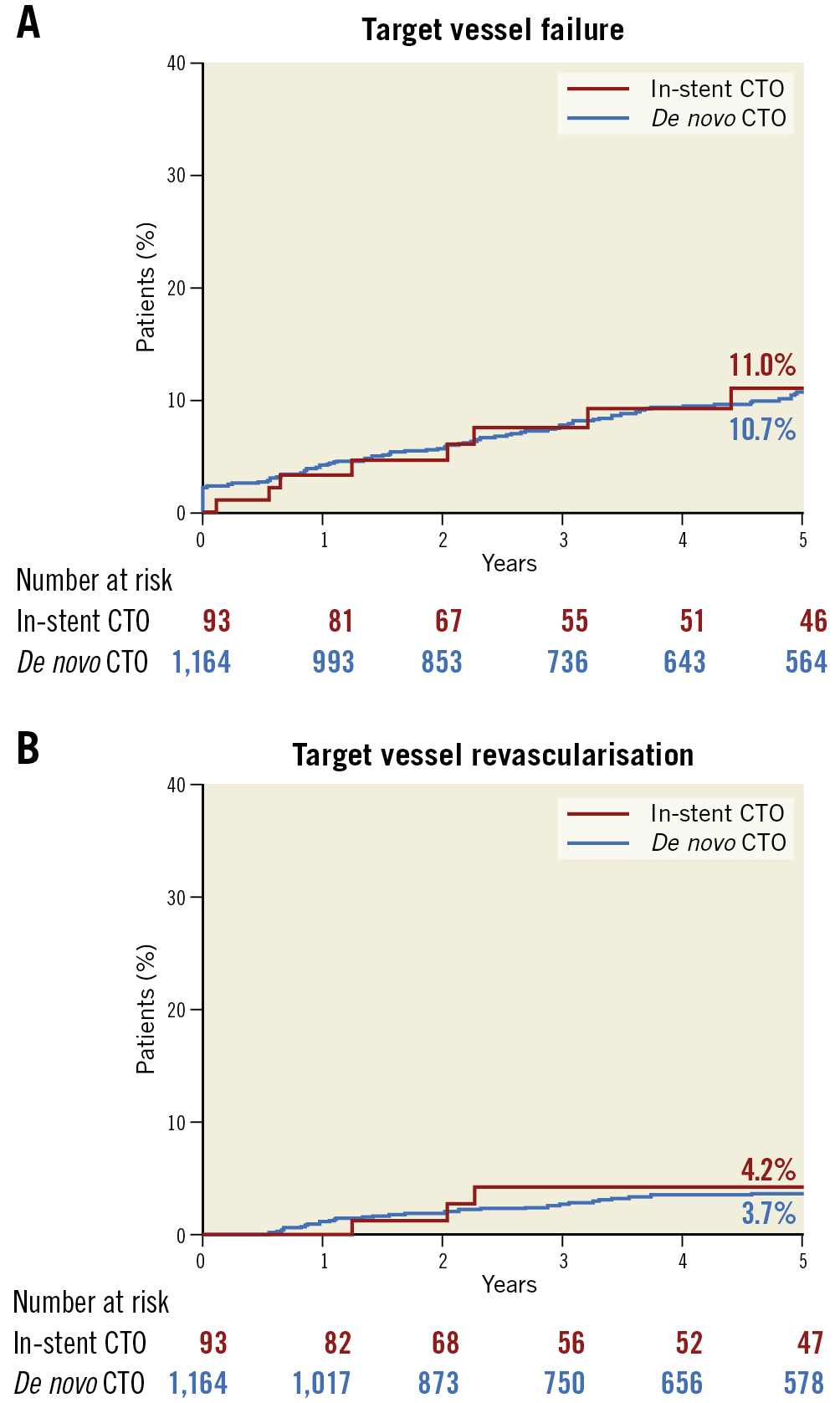
Figure 2. Kaplan-Meier event curves. A) Cumulative incidence of target vessel failure. B) Target vessel revascularisation.
Discussion
In this study we demonstrated that in-stent CTOs comprised approximately 10% of all the CTO PCI procedure cases and are characterised by less calcification but longer occlusion length with a similar overall CTO complexity and success rate compared with those for de novo CTOs. Among patients who underwent successful DES placement, the device-oriented composite endpoint of TVF was comparable between in-stent and de novo CTOs at five years, which should be viewed in the light of similar stent length and optimisation profiles achieved with IVUS guidance.
The prevalence of PCI for in-stent CTO varies from 5% to 25% in the published literature2,3,4,5,6. Perhaps technical difficulties to open the CTO effectively in an already stented segment and concerns about the long-term results after PCI are the main reasons why operators hesitate to attempt PCI for in-stent CTOs. Indeed, a recent analysis of a multicentre registry reported similar procedural success rates between in-stent and de novo CTO groups but a higher risk of adverse events during follow-up in the in-stent CTO group despite similar stent numbers and lengths6. However, nearly 90% of the total procedures were guided by angiography, and information regarding stent optimisation was unavailable. We believe that our report, which predominantly involves IVUS-guided CTO PCI, offers a practical guide for ensuring favourable long-term PCI results for in-stent CTOs.
The observation of comparable five-year post-stenting TVF rates between in-stent and de novo CTOs in our study could be reasonably explained by their comparable post-PCI IVUS parameters. The minimal stent area achieved in the two groups was notably similar. Accordingly, there was no difference in the frequency of stent underexpansion regardless of the definition used for the underexpansion. Moreover, the statuses of inflow or outflow track disease such as plaque burden and dissections were largely comparable. Because stent underexpansion and incomplete lesion coverage are well-known procedural factors responsible for stent failure, the association observed between the results of post-stenting IVUS measurements and clinical outcomes in our study seems plausible and is consistent with those of previous IVUS studies10,11,12.
A practical implication of our analysis is that, even in cases in which CTO segments are surrounded by prior stents, operators would be able to optimise stent expansion and location such as that achieved in de novo CTOs with IVUS guidance. In previous studies, IVUS-supported PCI, compared with angiography-guided PCI, was associated with a significant reduction in major adverse cardiac events by decreasing the propensity of suboptimal stent implantation13. The contributor to this benefit seemed to be the larger minimal lumen diameter or area associated with IVUS over angiography guidance14,15,16,17. Considering the proximal and distal reference vessel diameter of ~3.0 mm and ~2.0 mm, respectively, in the post-PCI quantitative coronary angiographic analysis in our cohort, the average stent diameter of 3.2 mm used may be a result of IVUS guidance14. Together with somewhat aggressive stent sizing and mandatory use of adjunctive post-dilation guided by IVUS, we were able to achieve similar minimal stent area between in-stent and de novo CTOs. However, it would also be important to emphasise that a substantial proportion of patients still had stent underexpansion by IVUS criteria despite these strategies, probably because of the inherent features of CTOs associated with high atherosclerotic burden, site calcifications, and lesions frequently extended to distal vessels. In other words, with PCI guided by angiography alone, the propensity of suboptimal stenting may substantially increase, leading to adverse stent-related events. Because previous studies have demonstrated that the benefit of IVUS-guided PCI is pronounced in more complex coronary anatomies such as long lesions, left main lesions, and CTOs14,15,16,17, the prognostic effect of this adjunctive tool may be maximised in in-stent CTOs. As advances in treatment algorithms and accumulating experience have increased the technical success of CTO PCI in recent years18,19,20, increasing numbers of patients with in-stent CTO will undergo PCI. As long as operators consider that the optimal stenting versus wire-crossing strategy may more significantly affect stent-related outcomes after CTO PCI, the long-term outcomes of PCI will maintain it as an effective treatment option for patients with in-stent CTO.
Limitations
This study has several limitations. First, it was retrospective and observational in nature; thus, it was inherently subject to bias. Second, the number of in-stent CTO patients evaluated using IVUS and for the outcome analysis was relatively small. More robust evidence incorporating a larger number of patients is necessary. Third, the relatively lower clinical risk profile of our study population and a mandatory exclusion of patients with a target CTO vessel diameter of <2.5 mm may have contributed to the low TVF rates and the overall study results21. Fourth, our study focused on patients who received stent-based treatment; extrapolating our findings to all patients with in-stent CTOs would be inappropriate. The final treatment strategy of in-stent CTO is determined considering various anatomical and technical factors such as the mechanisms of prior stent failure, presence of disease extension beyond the prior stent, or the degree of negative remodelling or wire-based dissection of the outflow vessels22,23. Accordingly, a non-stenting strategy for in-stent CTOs is commonly used in reality, which is in line with the proportion seen in our registry (non-stenting treatment was used in 22.9% of the overall in-stent CTO cohort vs 4.7% of the de novo CTO cohort). However, because procedural optimisation using a drug-coated balloon for in-stent restenosis seems relevant24, whether this concept is valid in in-stent CTO should be the subject of a future investigation.
Conclusions
The success and complication rates of contemporary PCI for in-stent CTOs are comparable to those for de novo CTOs. PCI with DES, optimised by IVUS guidance, offers acceptable long-term clinical results for in-stent CTOs.
|
Impact on daily practice Previously, in-stent CTO PCI was related with lower success rates and worse long-term outcomes compared with de novo CTOs. However, our study showed a similar success rate and in-hospital outcome in both CTO groups. In addition, the long-term results of PCI were also comparable between in-stent CTO and de novo CTO, which is achieved by IVUS-guided optimisation with drug-eluting stents. Therefore, our data support IVUS-guided PCI for in-stent CTO. |
Funding
This research was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI) funded by the Ministry of Health & Welfare, Republic of Korea (HI18C0846).
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

