Abstract
Background: Percutaneous coronary intervention (PCI) for chronic total coronary occlusions (CTO) improves clinical symptoms and quality of life. The longer-term safety of PCI compared to optimal medical therapy (OMT) remains uncertain.
Aims: We sought to evaluate the long-term safety of PCI for CTO in a randomised trial as compared to OMT.
Methods: A total of 396 patients with a symptomatic CTO were enrolled into a randomised, multicentre clinical trial comparing PCI and OMT. Half of the patients had a single CTO; the others had multivessel disease. Non-CTO lesions were treated prior to randomisation (2:1 ratio). During follow-up, crossover from OMT to PCI occurred in 7.3% (1 year) and 17.5% (3 years) of patients.
Results: At 3 years, the incidence of cardiovascular death or nonfatal myocardial infarction was not significantly different between the groups (OMT 3.7% vs PCI 6.2%; p=0.29). By per-protocol analysis, the difference remained non-significant (OMT 5.7% vs PCI 4.7%; p=0.67). Overall, major adverse cardiovascular events (MACE) were more frequent with OMT (OMT 21.2% vs PCI 11.2%), largely because of ischaemia-driven revascularisation. The rates of stroke or hospitalisation for bleeding were not different between the groups.
Conclusions: At 3 years there was no difference in the rate of cardiovascular death or myocardial infarction between PCI or OMT among patients with a remaining single coronary CTO. The MACE rate was higher in the OMT group due largely to ischaemia-driven revascularisation. CTO PCI appears to be a safe option for patients with a single remaining significant coronary CTO. CinicalTrials.gov: NCT01760083.
Introduction
Chronic coronary total occlusions (CTOs) represent a lesion subset found in about 15-20% of patients with stable coronary artery disease1. Recent advances in the treatment of CTOs have resulted in improved procedural success, but specific techniques and expertise are required2. It is widely accepted that the indication for CTO percutaneous coronary intervention (PCI) should be based on clinical symptoms rather than prognostic considerations3. The first phase of the EuroCTO trial demonstrated the benefit of PCI on freedom from angina and angina frequency over optimal medical therapy (OMT), with comparable 1-year safety4. However, the long-term safety of PCI for CTOs, or even a beneficial effect on adverse clinical events, is still under debate5.
Several registries and epidemiological studies have established the distinct negative effect of CTOs on prognosis, most prominently in patients with an acute coronary syndrome6 but also among patients with stable angina pectoris7. While registries have shown the benefit of a successful versus an unsuccessful PCI for CTO on prognosis8, the issue remains whether the failed procedure may have harmed the patient and biased the observation. On the other hand, comparisons of revascularised patients versus medically treated patients in registries involve selection bias on the part of the physician assigning the patient to the respective treatment options910. Propensity matching may partly correct this bias but certainly cannot replace a randomised comparison between PCI and OMT to understand the true impact of successful, failed, and deferred PCI on the clinical course of patients with a CTO. The design of the randomised EuroCTO trial included a 3-year follow-up period. The trial was not powered to detect differences in mortality, but, together with present and future randomised trials and registries, it was expected to offer insight into the safety of CTO PCI over a long-term period.
Methods
The design and rationale of this trial (ClinicalTrials.gov: NCT01760083) has been presented in detail with the results of the primary efficacy endpoint4. Briefly, the EuroCTO trial was a prospective, randomised, multicentre, open-label, controlled clinical trial to assess, in a 2:1 allocation, PCI with a biolimus-eluting stent (BioMatrix; Biosensors Europe) plus OMT and OMT alone (Central illustration). Patients were recruited at 28 European centres with expertise in CTO PCI (Supplementary Appendix). The trial was managed and overseen by an independent clinical research organisation (Cardiovascular European Research Centre, Massy, France), with outcome data assessed and adjudicated by a clinical events committee (CEC). The study protocol was approved by the relevant ethical committees, and written informed consent was obtained from all patients.
Patients with evidence of ischaemia or clinical symptoms and at least 1 CTO in a major coronary artery with a vessel diameter greater or equal to 2.5 mm (AHA site map 1-3, 6, 7, 11)11 were enrolled.
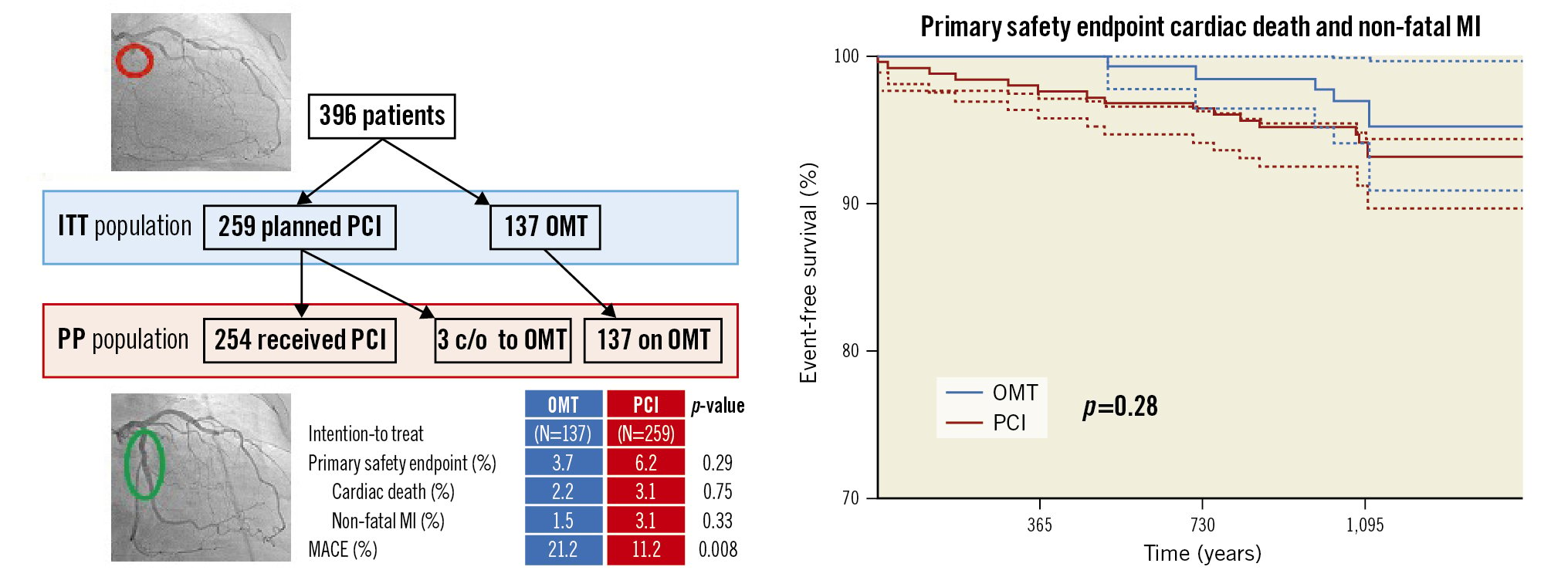
Central illustration. Study plan and 3-year outcome of the EuroCTO trial. At 3 years, there was no difference in the rate of cardiovascular death or non-fatal myocardial infarction between PCI or OMT among patients with a coronary CTO, while the MACE rate was higher with OMT. c/o: crossed over from 1 group to the other; ITT: intention-to-treat; MACE: major adverse cardiac events; MI: myocardial infarction; OMT: optimal medical therapy; PCI: percutaneous coronary intervention; PP: per protocol
Study intervention
Patients with a single-vessel CTO (48.5%) were treated according to randomisation. Patients with multivessel disease (51.5%) were included after at least 4 weeks from the completion of treatment of any other significant non-CTO lesions. In case of failed CTO PCI, additional interventional attempts were encouraged, including referral to bypass surgery. Angiographic success was defined as a final angiographic residual stenosis of <20% by visual estimate and Thrombolysis in Myocardial Infarction (TIMI) 3 flow after implantation of a drug-eluting stent. Dual antiplatelet therapy was advised for 12 months. Patients in both treatment groups received standard-of-care medication regarding lipid control, hypertension and diabetes treatment as well as medication in case of reduced left ventricular function as described in detail previously4. In the OMT group, antianginal medication was adapted during follow-up, and efforts were made to avoid unplanned revascularisation of the CTO in the OMT group before completion of the efficacy endpoint at 12 months. Thereafter, CTO recanalisation could be offered to patients randomised to the OMT arm with severe uncontrolled symptoms.
Primary safety endpoint
The primary safety endpoint was the incidence of cardiovascular death or non-fatal myocardial infarction (MI) during a follow-up of at least 3 years. Secondary endpoints were changes in the Canadian Cardiovascular Society (CCS) classification, major adverse cardiac events (MACE), stent thrombosis, cerebrovascular events, and hospitalisation for cardiac reasons.
Adverse event definition and assessment
MACE were defined as cardiac death, non-fatal MI, and ischaemia-driven target lesion revascularisation during follow-up. The latter was indicated by symptoms not controlled under optimised antianginal medication and assessed by the CEC as ischaemia-driven. Criteria for acute MI followed the third universal definition of MI12 and required the detection of a rise of cardiac biomarker values (preferably cardiac troponin with at least 1 value above the 99th percentile upper reference limit) combined with either symptoms of ischaemia, new or presumed new significant ST-segment/T-wave changes, new left bundle branch block, development of pathological Q waves in the electrocardiogram, new regional wall motion abnormality, or identification of an intracoronary thrombus by angiography or autopsy. Stent thrombosis was defined according to the Academic Research Council (ARC) criteria13.
Statistical Analysis
The final sample size calculation was based on the efficacy endpoint at 1 year, as previously indicated4. For the safety endpoint, the expected rate of cardiovascular death or MI at 3 years in the OMT arm was estimated to be about 11% and 8% in the PCI arm, according to registries and trials available at the time of the study design141516. For these estimates the non-inferiority hypothesis would have required about 1,200 patients for a power of 0.8, a sample size not achievable because of slow enrolment.
The primary analysis is based on the intention-to-treat (ITT) population. An additional per-protocol (PP) analysis was done excluding patients who had not received their assigned treatment. The mean and standard deviation are reported for continuous variables, and numbers and percentages are reported for categorical variables. Concomitant medication was summarised and compared between the groups at discharge and during follow-up by means of a chi-squared test or Fisher’s exact test, whichever was appropriate. The total number of antianginal drugs was compared by means of a Wilcoxon rank-sum test. The number of adverse events was compared between groups by means of a chi-squared test on the Kaplan-Meier estimates. All analyses were performed with the use of SAS, version 9.4 (SAS Institute). Further analyses beyond the ITT population evaluation were done with the use of NCSS 12 Statistical Software (NCSS).
Results
Enrolment and protocol adherence
Patients were enrolled over a 3-year period with 396 patients fulfilling the inclusion criteria; they were randomised 2:1 to either PCI and OMT (n=259) or OMT alone (n=137). This represents the ITT population. The main patient and lesion characteristics are summarised in Table 1. After randomisation, 5 patients did not undergo PCI (1 cancer diagnosis, 1 death from heart failure, 3 patients had a crossover to OMT by physician’s choice). Consequently, 254 patients underwent PCI, and 140 patients were treated by OMT alone, representing the per protocol population (Figure 1).
In the OMT group, 10 patients (7.3%) crossed over to PCI before completion of the 1-year follow-up. During the follow-up beyond 12 months, another 14 patients (10.2%) in the OMT arm underwent CTO revascularisation, with one of them directly referred to coronary artery bypass grafting (CABG). In addition, 8 patients in the PCI group and 3 in the OMT group withdrew their consent for further follow-up.
Table 1. Patient and lesion characteristics of patients with a CTO randomised to OMT or PCI.
| OMT (N=137) |
PCI (N=259) |
|
|---|---|---|
| Age, years | 64.7±9.9 | 65.2±9.7 |
| Male | 118 (86.1) | 215 (83.0) |
| Diabetes | 40 (29.2) | 85 (32.8) |
| Hypercholesterolemia | 111 (81.0) | 210 (81.1) |
| History of smoking | 92 (67.2) | 190 (73.4) |
| Previous myocardial infarction | 25 (18.3) | 59 (22.8) |
| Previous bypass surgery | 10 (7.3) | 34 (13.1) |
| Previous PCI unrelated to study | 71 (51.8) | 145 (56.0) |
| Previous PCI to facilitate study entry | 37 (27.0) | 79 (30.5) |
| Left ventricular ejection fraction, % | 55.7±10.8 | 54.5±10.8 |
| Single-vessel disease | 62 (45.3) | 130 (50.2) |
| J-CTO score | 1.67±0.91 | 1.82±1.07 |
| Numbers are mean±standard deviation or counts (%). There were no significant differences between the treatment groups. CTO: chronic total occlusion; J-CTO: Japanese chronic total occlusion score; OMT: optimal medical therapy; PCI: percutaneous coronary intervention | ||
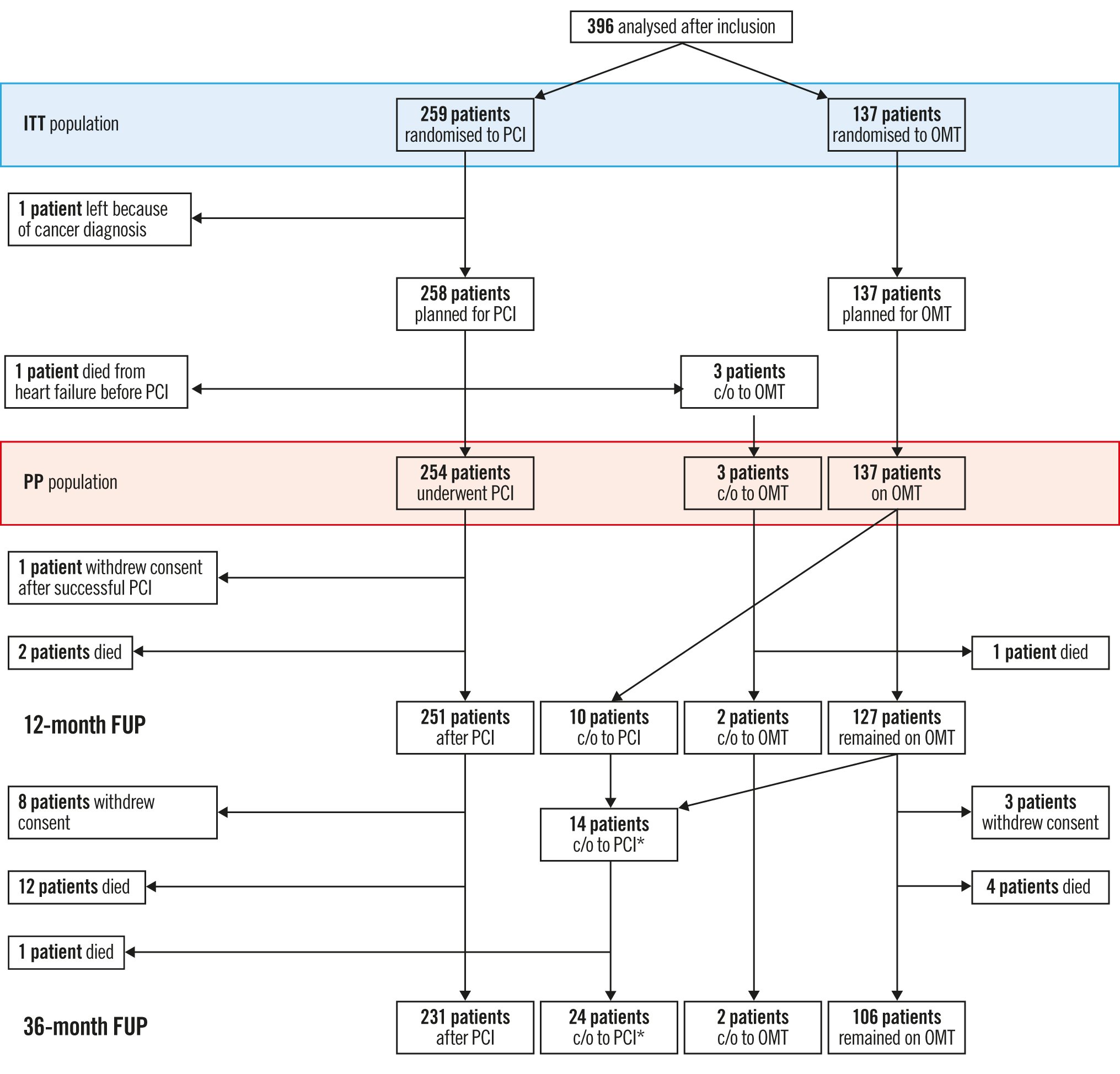
Figure 1. Overview of enrolment, treatment assignment and crossover of study patients during 3 years of follow-up. *1 patient crossed over to CABG directly. CABG: coronary artery bypass graft; c/o: crossed over from 1 group to the other; FUP: follow-up; ITT: intention-to-treat; OMT: optimal medical therapy; PCI: percutaneous coronary intervention; PP: per protocol
Procedural data of patients undergoing PCI
Recanalisation was attempted in 254 patients in the PCI group. PCI was successful in the first attempt in 83.1% of cases. Further attempts were made in 19 patients, of which 9 were successful, leading to a final success rate of 86.6% (Supplementary Figure 1). In-hospital complications occurred in 2.9% of patients, which included pericardial tamponade (n=4), vascular surgical repair (n=2) and need for a blood transfusion (n=5). There were no periprocedural deaths or emergency CABG.
The crossover procedures in 24 patients were successful in 19, unsuccessful in 4, and 1 patient was directly referred to CABG (Supplementary Figure 1). There were no periprocedural complications during these crossover PCIs. Finally, 278 patients underwent CTO revascularisation, two of them by CABG, and overall, 241 (87.3%) patients were successfully revascularised.
Clinical symptoms during follow-up
The CCS class and the New York Heart Association (NYHA) Functional Class improved at 12 months, more in the PCI group than in the OMT group. At baseline, there were 81.8% in CCS <2 as compared to 65.7% (p<0.001) at 12 months, and 75.8% versus 53.3% (p<0.001) in NYHA <2. At the final assessment, CCS <2 was observed in 77.3% vs 66.1% (p<0.05), while there was no longer a difference in NYHA <2 (71.1% vs 59.5%). The number of antianginal medications was significantly lower in the PCI group as compared to the OMT group at 12-month follow-up (1.4 vs 1.9; p<0.005), and this remained within the same range at 3 years (1.4 vs 1.8; p<0.05).
Clinical safety endpoint in the intention-to-treat and per-protocol ANALYSES
During a follow-up of 37.4±7.7 months, cardiovascular death or nonfatal MI were not significantly different in either group, with 3.7% in the OMT group and 6.2% in the PCI group (log-rank p=0.29). In the per-protocol analysis, 5.7% had an event in the OMT group and 4.7% in the PCI group (p=0.67) (Figure 2).
In the PCI group, there were 2 procedure-related MIs (0.8%) during unsuccessful recanalisation attempts. The spontaneous MI rate during follow-up was 2.3% in the PCI group and 1.5% in the OMT group, while for crossover procedures no periprocedural MI was detected.
The MACE rate was significantly higher in the OMT group, with 21.2% as compared to 11.2% in the PCI group, in the ITT analysis (p=0.008). The main contributor for MACE in the OMT group was ischaemia-driven revascularisation (Table 2). In the PP analysis, this difference was higher, with 22.9% in the OMT group and 9.8% in the PCI group (p<0.001) (Figure 3). In the PCI group, 9 patients (3.5%) with an initially successful procedure had a lesion recurrence requiring repeat PCI. In addition, there was 1 (0.7%) non-target revascularisation in the OMT group and 10 (3.9%) non-target revascularisations in the PCI group in patients either with disease progression in a previously untreated segment or recurrence in a previously treated non-CTO lesion.
There were 6 cerebral ischaemic events reported (all but 2 with full neurological recovery) 1 (0.7%) in the OMT group, 5 (1.9%; p=0.67) in the PCI group of which 1 (0.4%) was periprocedural. Three (1.2%) periprocedural bleeding events were reported, while during follow-up there was 1 (0.7%) bleeding event in the OMT group and 4 (1.5%) in the PCI group. Including the periprocedural events, there was no statistical difference (p=0.27) between groups.
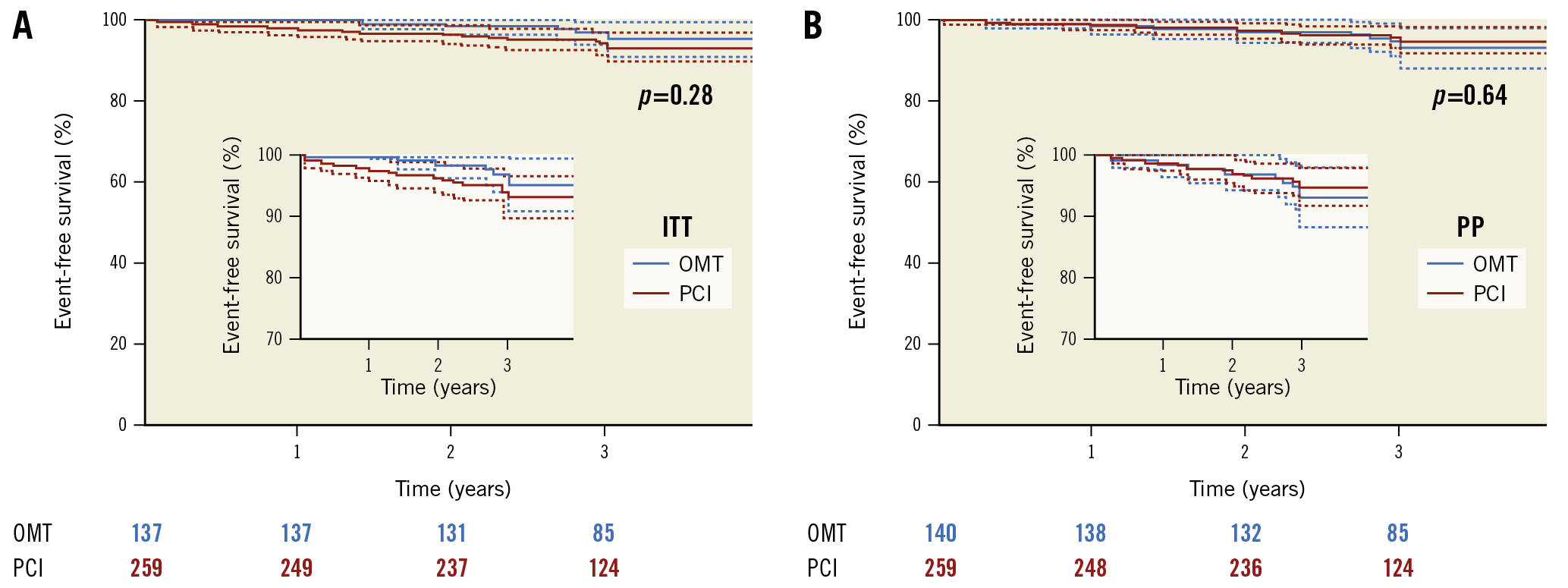
Figure 2. Kaplan-Meier survival plot, free of cardiovascular death and non-fatal MI, in patients assigned to PCI or OMT for a minimum follow-up of 3 years. A) Intention-to-treat analysis. B) Per-protocol analysis. ITT: intention-to-treat; OMT: optimal medical therapy; PCI: percutaneous coronary intervention; PP: per protocol
Table 2. Adverse events at follow-up in patients with a CTO randomised to OMT or PCI (intention-to-treat and per protocol)
| OMT | PCI | p-value | |
|---|---|---|---|
| Intention-to-treat | (N=137) | (N=259) | |
| Primary safety endpoint cardiac death and MI | 3.7 | 6.2 | 0.29 |
| Cardiac death | 2.2 | 3.1 | 0.75 |
| Non-fatal myocardial infarction | 1.5 | 3.1 | 0.33 |
| Non-cardiac death | 1.5 | 3.1 | 0.50 |
| MACE | 21.2 | 11.2 | 0.008 |
| Ischaemia-driven revascularisation | 17.5 | 7.3 | 0.002 |
| Ischaemia-driven target revascularisation | 16.8 | 3.5 | <0.001 |
| Stroke | 0.7 | 2.0 | 0.43 |
| Hospitalisation for bleeding | 0.7 | 2.8 | 0.27 |
| Per protocol | (N=140) | (N=254) | |
| Primary safety endpoint cardiac death and MI | 5.7 | 4.7 | 0.67 |
| Cardiac death | 2.9 | 2.4 | 0.76 |
| Non-fatal myocardial infarction | 2.9 | 2.4 | 0.76 |
| Non-cardiac death | 1.4 | 3.2 | 0.50 |
| MACE | 22.9 | 9.8 | <0.001 |
| Ischaemia-driven revascularisation | 18.6 | 6.7 | <0.001 |
| Ischaemia-driven target revascularisation | 16.4 | 3.5 | <0.001 |
| Stroke | 0.7 | 2.0 | 0.43 |
| Hospitalisation for bleeding | 0.7 | 2.8 | 0.27 |
| All data are presented as %. CTO: chronic total occlusion; MACE: major adverse cardiovascular events; MI: myocardial infarction; OMT: optimal medical therapy; PCI: percutaneous coronary intervention | |||
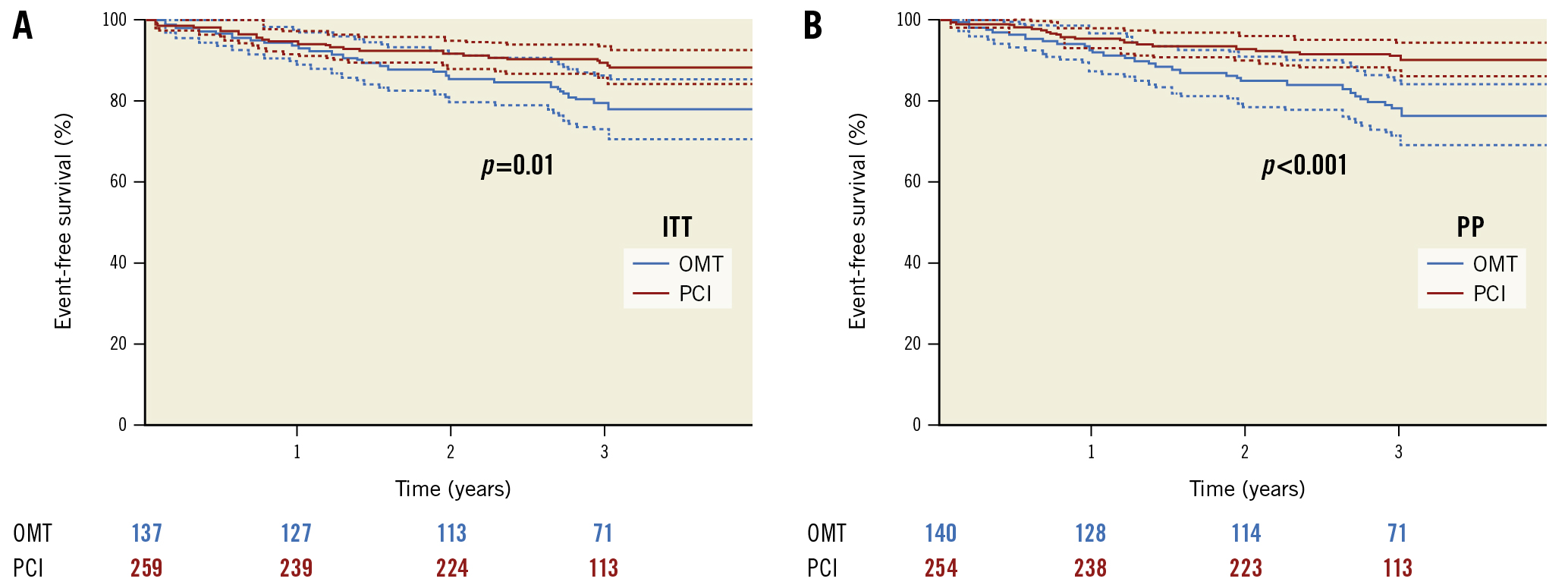
Figure 3. Kaplan-Meier survival plot, free of major adverse cardiac events (MACE) in patients assigned to PCI or OMT for a minimum follow-up of 3 years. A) Intention-to-treat analysis. B) Per-protocol analysis. ITT: intention-to-treat; OMT: optimal medical therapy; PCI: percutaneous coronary intervention; PP: per protocol
Comparison of patients with successful revascularisation versus failed procedures versus OMT
In patients with a successful PCI, the incidence of the primary safety endpoint was 3.6% versus 12.2% in those with failed PCI (p=0.03) and 5.7% in those on OMT at follow-up. Two of the events were periprocedural MIs, and 2 were cardiac deaths during further follow-up in the failed-PCI patients. The rate of MACE was lowest after successful PCI 8.6% versus 15.2% with unsuccessful PCI versus 22.9% in the OMT patients.
Discussion
In the first phase of this trial of patients with stable angina pectoris and at least 1 CTO, we found that, compared with medical treatment alone, CTO recanalisation led to better symptom control and improved quality of life. While in non-CTO lesions, the seminal COURAGE trial showed a similar benefit for PCI as compared to OMT; however, in that study, PCI appeared to be associated with more MI13. In the current prespecified long-term safety analysis after at least 3 years, we observed no increased risk of cardiovascular death or MI in either the ITT or PP analysis, with an increased rate of ischaemia-driven revascularisation and MACE in the OMT group (Central illustration).
Long-term events after PCI versus OMT in patients with a CTO
The success rate in our study was high (86.6% in the PCI group and 87.3% overall, including procedures in crossover patients), a confirmation of the efficacy of contemporary recanalisation techniques, and comparable to recent prospective trials involving CTO PCI, such as SYNTAX II17, DECISION-CTO18, and REVASC19. Of these trials, the only one comparable in the selection of patients was the DECISION-CTO trial, which also had a 3-year follow-up. In the EuroCTO trial the periprocedural complications were low, with no deaths. During follow-up, the 3-year cardiovascular mortality rate in the PCI group was 3.1%, which is slightly higher than in DECISION-CTO, but the rate of MI was lower, at 3.1% as compared to 11.3%. In the latter trial, there was a higher rate of periprocedural MI of 9.8%, whereas the rate of spontaneous MI was 1.7% in the PCI group. However, the OMT group of the DECISION-CTO trial cannot be compared to the present trial, as more than 70% of patients received a PCI for a non-CTO lesion, resulting in a comparably higher rate of periprocedural MI of 7.5%, with a spontaneous MI rate of 1.8%, similar to the PCI group.
In our study, the ITT analysis showed a numerically higher rate of the safety endpoint in the PCI group compared to the OMT group (6.2% vs 3.7%; p=0.29), while in the PP analysis there was a reverse numerical difference between the OMT group and the PCI group (4.7% vs 5.7%; p=0.67). The MACE rate was higher in the OMT group with 21.2% versus 11.2% (PCI group) (p<0.008), mainly due to the rate of ischaemia-driven revascularisations − despite the effort to avoid crossovers by the attending physicians − but also due to the low rate of repeat revascularisation in the PCI group (3.5%) during the 3-year follow-up.
Randomised trials versus registries to assess long-term outcome after CTO PCI
Both our study and the DECISION-CTO trial demonstrated that patients undergoing PCI for a CTO do not have a significantly higher rate of death or MI than patients treated by OMT. As neither study was powered to detect a difference between the 2 strategies for this safety endpoint, the results do not support the observations of registries in which PCI seemed to be superior to OMT regarding death and MI202122. A recently published registry with a 10-year follow-up indicated that the follow-up period must be extended well beyond 5 years to detect an influence on hard clinical endpoints9.
An inherent problem of all randomised trials in this field is the exclusion of highly symptomatic patients. Both patients and referring physicians will decline to enrol in a trial with a conservative option when the alternative would be interventional therapy with immediate relief of symptoms23. Therefore, lower-risk patients are enrolled, as shown in a systematic analysis by Megaly et al24.
The importance of successful procedures for long-term outcome
Even with no randomised trial supporting a prognostic impact of CTO PCI, the beneficial effect on clinical symptoms is a sound indication to perform PCI in order to avoid an additional lifelong antianginal medication. As shown in our study, there were no significant risks added by the procedure. In the PP analysis looking at successful and unsuccessful CTO procedures, procedural success is a major determinant of the clinical course after PCI. This is already established in the many registries comparing successful and unsuccessful CTO procedures25, but it is emphasised once again by our observations that an optimal result is important to achieve a favourable clinical outcome after CTO PCI26.
Limitations
This study was not powered to detect superiority in the safety endpoint but was designed to detect changes in clinical symptoms in both treatment groups. The number of randomised patients did not reach the prespecified target for the primary efficacy endpoint due to the limited number of expert centres and the hesitation to randomise highly symptomatic patients. The limitation to expert centres was chosen to avoid low procedural success rates. Non-expert centres would have affected the assessment of the procedural benefit as failed procedures carry a higher complication rate and would bias the results. This can also be observed when comparing successful and unsuccessful PCI in our study.
The event rate in both arms of this trial was lower than expected and much lower than the reported rates in large CTO registries and meta-analyses. This appears to be an unavoidable consequence of the selection process for enrolment in PCI studies, not limited to CTO PCI, but also including larger contemporary revascularisation versus OMT therapy trials such as ISCHEMIA27 or REVIVED-BCIS228. This weakens the applicability of the findings of these randomised studies to the overall population considered for PCI, which includes patients with more severe symptoms, more comorbidities and higher MACE rates24. The lower MACE rate observed in randomised trials as compared to published registries highlights the bias involved in recruiting patients into a trial in which interventional therapy is offered as one option instead of a conservative approach.
The analysis of the primary efficacy endpoint demonstrated a significant benefit in health status after PCI for a CTO as compared to OMT4. This is now supported by the safety endpoint analysis after 3 years which shows that this treatment effect was achieved without a higher risk of cardiovascular mortality and nonfatal MI. While current guidelines are critical of the indication to perform PCI for relief of symptoms and would rather suggest a lifelong antianginal therapy29, with no evidence of prognostic benefit, the EuroCTO trial demonstrated the superiority of PCI over OMT without impacting the patient’s acute and long-term safety. However, the power of this study to detect significant differences in the clinical endpoints was limited, but the results are comparable to the DECISION-CTO trial18. Unlike the DECISION-CTO trial, the EuroCTO trial examined the specific effect of CTO PCI versus OMT, whereas the former diluted the effect of CTO PCI by having extensively performed non-CTO PCI in the “OMT group”. In addition, a recent smaller randomised trial supported the initial findings of the EuroCTO trial regarding better symptom control30. To resolve this conflicting interpretation of data, the ongoing ISCHEMIA-CTO Trial will add further substance to the comparison between intervention and OMT regarding symptomatic improvement and MACE31.
Conclusions
The current study shows that PCI did not carry significantly higher risks of cardiovascular death and nonfatal MI during a follow-up of 3 years as compared to OMT in patients with a CTO, whereas MACE increased in the OMT group. Further analysis suggests that a successful PCI leads to a lower event rate during follow-up, while the worst outcome is observed in patients with failed PCI. Therefore, high procedural quality and success rates are mandatory when considering PCI as a primary option for symptom relief in patients with a CTO. Referral to expert centres should be considered for complex CTO lesions.
Impact on daily practice
The EuroCTO trial demonstrated a benefit of PCI regarding clinical symptoms as compared to OMT after 1 year. The further follow-up of this study showed sustained benefit regarding angina control, no increased risk of cardiovascular death or non-fatal MI, with lower MACE as compared to OMT, provided the procedure was successful. In patients with a symptomatic CTO, PCI can be offered as a safe option to avoid continued antianginal medication.
Dedication
This manuscript is dedicated to the memory of Professor Anthony Gershlick, who was one of the initiators of this trial and who succumbed to COVID-19 during his dedicated service as an interventional cardiologist.
Acknowledgements
We express our gratitude to Dr Marie-Claude Morice for the continued support of this study project.
Funding
The study was supported by an unrestricted grant from Biosensors Europe SA and Asahi Intecc Co., Ltd. The sponsor of the study was the EuroCTO Club e.V..Conflict of interest staG. Werner reports speaker honoraria from Asahi Intecc, Abbott Vascular, Daiichi Sankyo, OrbusNeich, Philips/Volcano, Siemens, and Terumo. J. Escaned reports speaker honoraria from Abbott and Boston Scientific; and received honoraria from Philips. A. Erglis reports speaker fees from Biosensors, Biotronik, and Boston Scientific. E.H. Christiansen received research grants from Asahi Intecc and Biosensors.C. Di Mario reports institutional grants from Abbott Vascular, Amgen, Chiesi, Daiichi Sankyo, Edwards Lifesciences, Medtronic, Philips, and Shockwave; and personal speaker honoraria from Shockwave and Philips/Volcano. A. Bufe reports speaker honoraria from Biotronik. B. Lauer reports speaker honoraria from Daiichi Sankyo, Amgen, Nicolai, Asahi Intecc, Abbott, and Abiomed. The other authors have no conflict of interest to declare.
Conflict of interest statement
G. Werner reports speaker honoraria from Asahi Intecc, Abbott Vascular, Daiichi Sankyo, OrbusNeich, Philips/Volcano, Siemens, and Terumo. J. Escaned reports speaker honoraria from Abbott and Boston Scientific; and received honoraria from Philips. A. Erglis reports speaker fees from Biosensors, Biotronik, and Boston Scientific. E.H. Christiansen received research grants from Asahi Intecc and Biosensors.C. Di Mario reports institutional grants from Abbott Vascular, Amgen, Chiesi, Daiichi Sankyo, Edwards Lifesciences, Medtronic, Philips, and Shockwave; and personal speaker honoraria from Shockwave and Philips/Volcano. A. Bufe reports speaker honoraria from Biotronik. B. Lauer reports speaker honoraria from Daiichi Sankyo, Amgen, Nicolai, Asahi Intecc, Abbott, and Abiomed. The other authors have no conflict of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

