Abstract
Chronic total occlusions (CTOs) of coronary arteries can be found in the context of chronic or acute coronary syndromes; sometimes they are an incidental finding in those apparently healthy individuals undergoing imaging for preoperative risk assessment. Recently, the invasive management of CTOs has made impressive progress due to sophisticated preinterventional assessment, including advanced non-invasive imaging, the availability of novel and dedicated tools for CTO percutaneous coronary intervention (PCI), and experienced interventionalists working in specialised centres. Thus, it is crucial that referring physicians who see patients with CTO be aware of recent developments and of the initial evaluation requirements for such patients. Besides a careful history and clinical examination, electrocardiograms, exercise tests, and non-invasive imaging modalities are important for selecting the patients most suitable for CTO PCI, while others may be referred to coronary artery bypass graft or optimal medical therapy only. While CTO PCI improves angina and reduces the use of antianginal drugs in patients with symptoms and proven ischaemia, hibernation and/or wall motion abnormalities at baseline or during stress, the effect of CTO PCI on major cardiovascular events is still controversial. This clinical consensus statement specifically focuses on referring physicians, providing a comprehensive algorithm for the preinterventional evaluation of patients with CTO and the current evidence for the clinical effectiveness of the procedure. The proposed care track has been developed by members and with the support of the European Association of Percutaneous Cardiovascular Interventions (EAPCI), the European Association of Cardiovascular Imaging (EACVI), and the European Society of Cardiology (ESC) Working Group on Cardiovascular Surgery.
The management of coronary chronic total occlusions (CTOs) is clinically and technically challenging and requires a close collaboration between referring physicians and specialised centres1. The therapeutic options for patients with CTOs have expanded immensely thanks to sophisticated preinterventional planning, including advanced cardiac imaging, advanced percutaneous coronary intervention (PCI) equipment, cardiac surgery, and effective anti-ischaemic optimal medical therapy (OMT). Because of these developments, the success rate of CTO PCI today exceeds 80-90% in the hands of expert operators working at specialised CTO referral centres23. According to the CTO Academic Research Consortium (CTO-ARC), a CTO is defined as an occlusion of an epicardial coronary artery without antegrade flow through the lesion and with a probable or definite duration of ≥3 months, based on angiographic criteria such as a Thrombolysis in Myocardial Infarction (TIMI) grade 0 flow through the lesion with no evidence of a thrombus, no staining at the proximal cap, and the presence of mature collaterals4. Contemporary, consecutive series of patients undergoing invasive coronary angiography (ICA) reported the presence of at least one CTO in 15-20% of cases56.
However, the decision-making process for the management of such patients requires a thorough clinical evaluation with initial examinations including electrocardiograms (ECGs), echocardiography, exercise tests, or cardiac imaging stress tests.
This clinical consensus statement, involving the European Society of Cardiology (ESC) Association of Percutaneous Cardiovascular Interventions (EAPCI), the ESC Association of Cardiovascular Imaging (EACVI), as well as the ESC Working Group on Cardiovascular Surgery, proposes a comprehensive algorithm for patients in whom a CTO has been diagnosed, providing a preinterventional evaluation targeted to the patient’s condition and needs.
The CTO patient: characteristics and clinical phenotypes
CTOs may be discovered in different clinical settings, such as acute coronary syndromes (ACS), in the context of chest pain evaluation, as a consequence of documented ischaemia using different imaging modalities1 or incidentally, during a coronary angiography workup before surgical valve replacement or vascular surgery, among others156. While patients complaining of exertional symptoms (e.g., angina, dyspnoea) have a clear diagnostic and therapeutic path to be followed, asymptomatic patients must be carefully evaluated based on further examinations usually not available to the treating physician at the time of presentation (Figure 1).
In both circumstances, it is desirable to carefully evaluate the patient’s age, frailty, and comorbidities (e.g., significant concomitant valvular heart disease, large aortic aneurysms, non-cardiac limitations of functional capacity, ongoing oncological treatment and/or cognitive deficits, among others). These clinical elements should concur with technical considerations to guide the decision between OMT or revascularisation and, in the latter group, between PCI and surgery. Of note, patients with CTO are older, more often diabetic and with a greater impairment of left ventricular ejection fraction (LVEF), compared with patients without CTO6.
Other patients may present with ACS, including ST-segment elevation myocardial infarction (STEMI). In the case of type 1 myocardial infarction (MI), with either a plaque rupture or erosion in the culprit artery providing collaterals to another coronary artery with a CTO, prognosis is poor because of the double injury due to interruption of collateral flow from the culprit artery to the CTO territory7. Notably, about one-third of patients who are resuscitated because of a cardiac arrest have a CTO8. If severe acute ischaemia has led to cardiogenic shock, complete revascularisation beyond the infarct-related artery should be avoided. Indeed, the initial suggestion of the SHOCK trial that complete revascularisation should be attempted in this setting was refuted by later trials910. Conversely, there is convincing evidence supporting complete revascularisation for STEMI without cardiogenic shock and multivessel disease11. However, these trials were not specifically targeted for patients with CTO in non-culprit arteries (Table 1).
Patients with non-STEMI-ACS also require immediate treatment of the culprit artery, with the added challenge of ruling out the CTO as the cause of or contributor to the acute event12 (Table 1). Besides clinical clues, the absence of contrast staining, which is typical of fresh thrombotic occlusions, and the presence of well-developed collaterals towards the distal segment of the CTO normally allow a distinction between acute and chronic coronary occlusions. A particularly interesting subgroup includes patients with type 2 MI (i.e., MI secondary to ischaemia due to either increased oxygen demand or decreased supply). They typically present with increased troponin levels, ECG changes and/or regional wall motion abnormalities, with or without symptoms113. In such cases, the treatment should focus on the acute trigger disrupting a previously stable situation with no immediate need of an urgent or emergent CTO revascularisation, but with consideration of it at a later stage (Table 1).
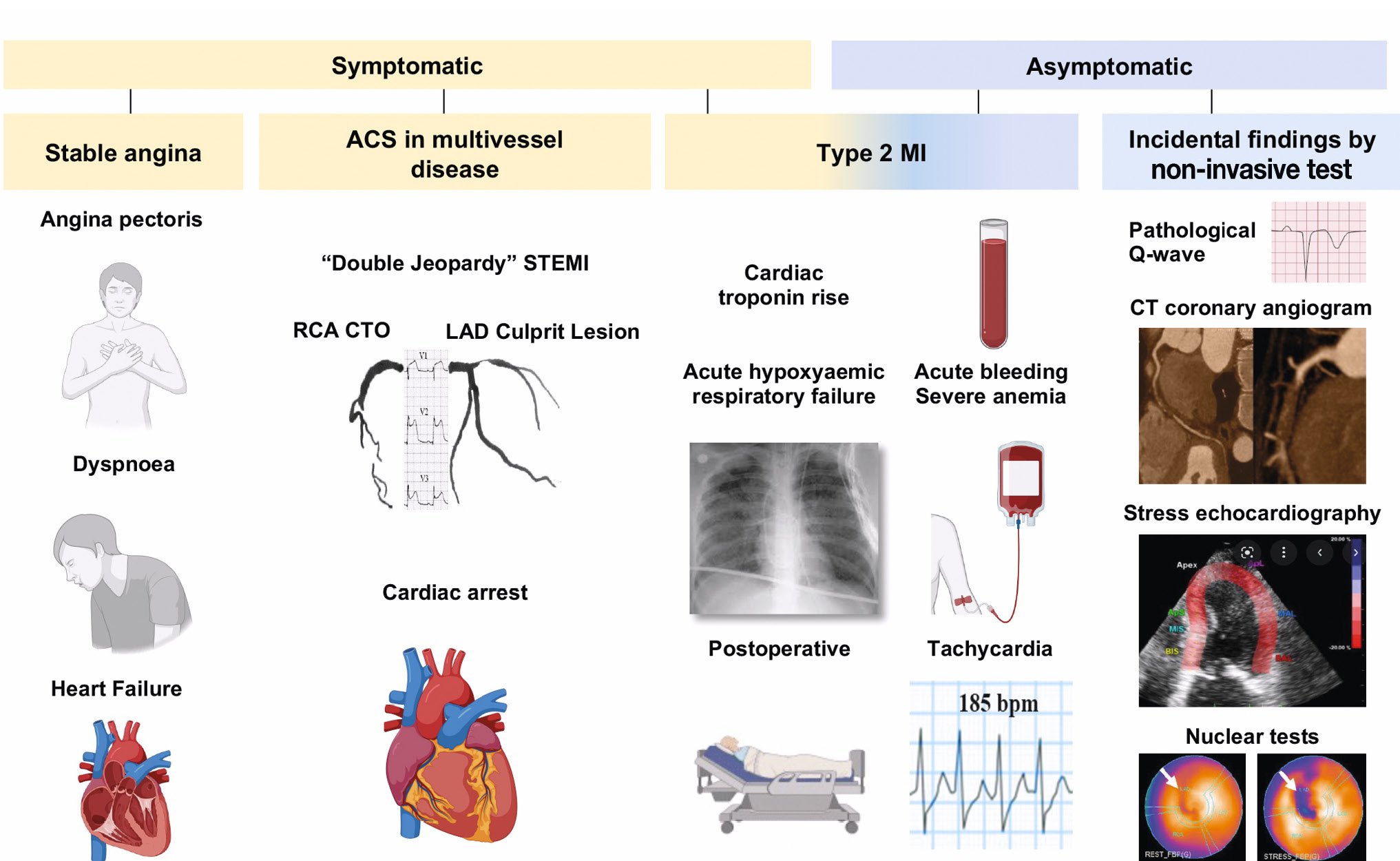
Figure 1. Different clinical presentation modalities of patients with coronary CTO. The CTO may be discovered in symptomatic patients in the context of stable angina with or without signs/symptoms of heart failure, ACS, troponin rise and/or ECG/echocardiographic changes during supraventricular arrhythmias, after acute bleedings or during/after a non-cardiac operation (type 2 MI). In asymptomatic patients, a CTO may be detected as an incidental finding after CTCA or a positive stress test. ACS: acute coronary syndrome; CT: computed tomography; CTCA: computed tomography coronary angiography; CTO: chronic total occlusion; ECG: electrocardiogram; LAD: left anterior descending artery; MI: myocardial infarction; RCA: right coronary artery
Table 1. Expert panel statements.
| Evidence available | |
|---|---|
| The CTO recanalisation success rate has dramatically increased (85-90%), provided that expert operators are offered the full availability of dedicated interventional tools. | RCTs and registries: uncontroversial235625262948495455565960 |
| Frequency and severity of complications is higher (1-3%) compared with most PCI procedures in CCS. This requires careful consideration of the benefit and risk balance before embarking upon CTO recanalisation or moving to recanalisation modalities (ADR, retrograde) that pose higher complication risks. | Registries and meta-analyses: uncontroversial2356254849 |
| A specific consent form listing the differences with other PCI procedures (duration, double access, lack of certainty of success, slightly higher risk of complications) should be submitted to patients and discussed with the main operator before the procedure. | Expert consensus |
| Ad hoc CTO PCI (i.e., during the same diagnostic angiogram) is discouraged. CTO PCI should be started only after having ensured that sufficient time is available, experienced operators are present and a well-defined strategy has been developed. The treating physicians will have the opportunity to review indications, perform additional tests if needed, and inform the patient and his family, leaving enough time for decision-making. | Expert consensus |
| The complexity of CTO procedures can be graded, and the most complex (stumpless, ostial, very calcified, or long and tortuous, previously failed) should be reserved for dedicated operators or performed with proctorship. | Expert consensus |
| The presence of a CTO during ACS (especially STEMI) increases the risk that the patient develops cardiogenic shock, but attempts at recanalisation in the acute phase should be discouraged. | Randomised trials91011 |
| Complete revascularisation appears beneficial in STEMI and, with less compelling evidence, in NSTEMI. CTO PCI during primary angioplasty should be discouraged. | Randomised trial11. Expert consensus on timing and modalities of CTO treatment |
| In CCS patients with multivessel disease with clinical or anatomical preference for PCI over CABG, the timing of CTO PCI and the sequence of treatment of non-CTO and CTO lesions deserve careful consideration. | Expert consensus |
| Symptoms of angina or dyspnoea likely caused by the persistence of a CTO and resistant to medical therapy should be the main driver of CTO recanalisation. | Randomised trials and registries5152535455 |
| Myocardial revascularisation decision, in the context of left main/multivessel disease, including CTO lesions, is optimised by a Heart Team approach. | ESC and ACC guidelines on myocardial revascularisation4344 |
| Current evidence does not support the use of CTO PCI to improve prognosis (reduce mortality and incidence of myocardial infarction). Randomised trials have limitations in terms of sample size, patient selection bias and trial design, and it is worth noting the opposite results in large, nationwide, long-term registries comparing patients who had successful or failed CTO with those patients undergoing revascularisation or left under medical treatment. | Consensus that the conflicting evidence from randomised trials and large registries5152535455565758596061 is still insufficient to draw firm conclusions |
| Current evidence is still not sufficient to draw firm conclusions on the use of CTO PCI to improve left ventricular function. | Evidence from meta-analyses24 and registries2532 was not confirmed in a randomised64 trial |
| The assessment of inducible ischaemia and viability in the CTO territory is advisable in case of LV dysfunction. MRI, if available, should be preferred over nuclear tests and stress echocardiography for viability assessment. | Expert consensus |
| ACC: American College of Cardiology; ACS: acute coronary syndromes; ADR: antegrade dissection and re-entry; CABG: coronary artery bypass grafting; CCS: chronic coronary syndrome; CTO: chronic total occlusion; ESC: European Society of Cardiology; LV: left ventricular; MRI: magnetic resonance imaging; NSTEMI: non-ST-segment elevation myocardial infarction; PCI: percutaneous coronary intervention; RCT: randomised controlled trials; STEMI: ST-segment elevation myocardial infarction | |
Initial examination
The main indication for CTO recanalisation is to relieve exercise-limiting symptoms such as angina. Thus, the initial clinical examination requires a comprehensive and careful assessment of the symptomatic status, as well as possible changes in the customary exercise levels over the past months or years, taking in account that, often, CTO patients may adapt to their limited exercise capacity and may not perceive or report their functional status limitation appropriately. Furthermore, symptoms are not limited to chest pain only; indeed, dyspnoea is at least as frequent as angina, and this improves after successful recanalisation14.
The assessment of exercise-induced symptoms can be objectively performed in a “patient-centred” fashion, using tools such as the modified Seattle Angina Questionnaire (SAQ)15, the EuroQol quality-of-life 5-dimensional score (EQ-5D)16 or Rose Dyspnea Scale (RDS)17 (Figure 2). Depression may also be more prevalent in patients with CTO18 and may mask exercise-related symptoms. After blood tests, ECGs and echocardiography, different imaging modalities are useful to assure that an angiographically documented CTO serves viable myocardium and indeed induces significant ischaemia during stress19 (Central illustration). If an imaging stress test is not feasible or locally unavailable, a bicycle or treadmill exercise tolerance test might be carried out to determine the patient’s true functional capacity, the severity of exercise-induced angina and/or dyspnoea, and their reproducibility compared to the patient’s history.
Furthermore, many patients may have silent ischaemia in the CTO territory as well. In such cases, collaterals commonly prevent regional myocardial dysfunction or MI, but their functional capacity to increase myocardial blood flow to the CTO territory during exercise may be limited. Typically, fractional flow reserve (FFR) assessed distal to a CTO is usually below 0.52021. Thus, the use of non-invasive ischaemia tests should be strongly encouraged, especially in patients with atypical symptoms or in those complaining of dyspnoea, to ascertain their ischaemic origin. Finally, in asymptomatic patients with left ventricular (LV) dysfunction, the assessment of inducible ischaemia/viability may have a prognostic value22 and might be considered during follow-up to guide coronary revascularisation in selected cases only (Central illustration).
If ischaemia leads to regional wall motion abnormalities, as assessed by imaging, the functional recovery of LV function depends on the extent of hibernating or stunned, but viable, myocardium. However, the evaluation of hibernation or stunning in patients with CTO has led to conflicting results23242526 (Table 1).
One aspect that is often not routinely evaluated is the incidence of ventricular arrhythmias (VA) in patients with CTO and ischaemic cardiomyopathy. Indeed, among patients with VA on admission, the presence of a coronary CTO is independently associated with increased midterm all-cause mortality27. Furthermore, among patients with ischaemic cardiomyopathy and an implantable cardioverter-defibrillator (ICD) for secondary prevention of sudden cardiac death (SCD), the presence of an angiographically documented CTO is an independent predictor of appropriate ICD therapy28.
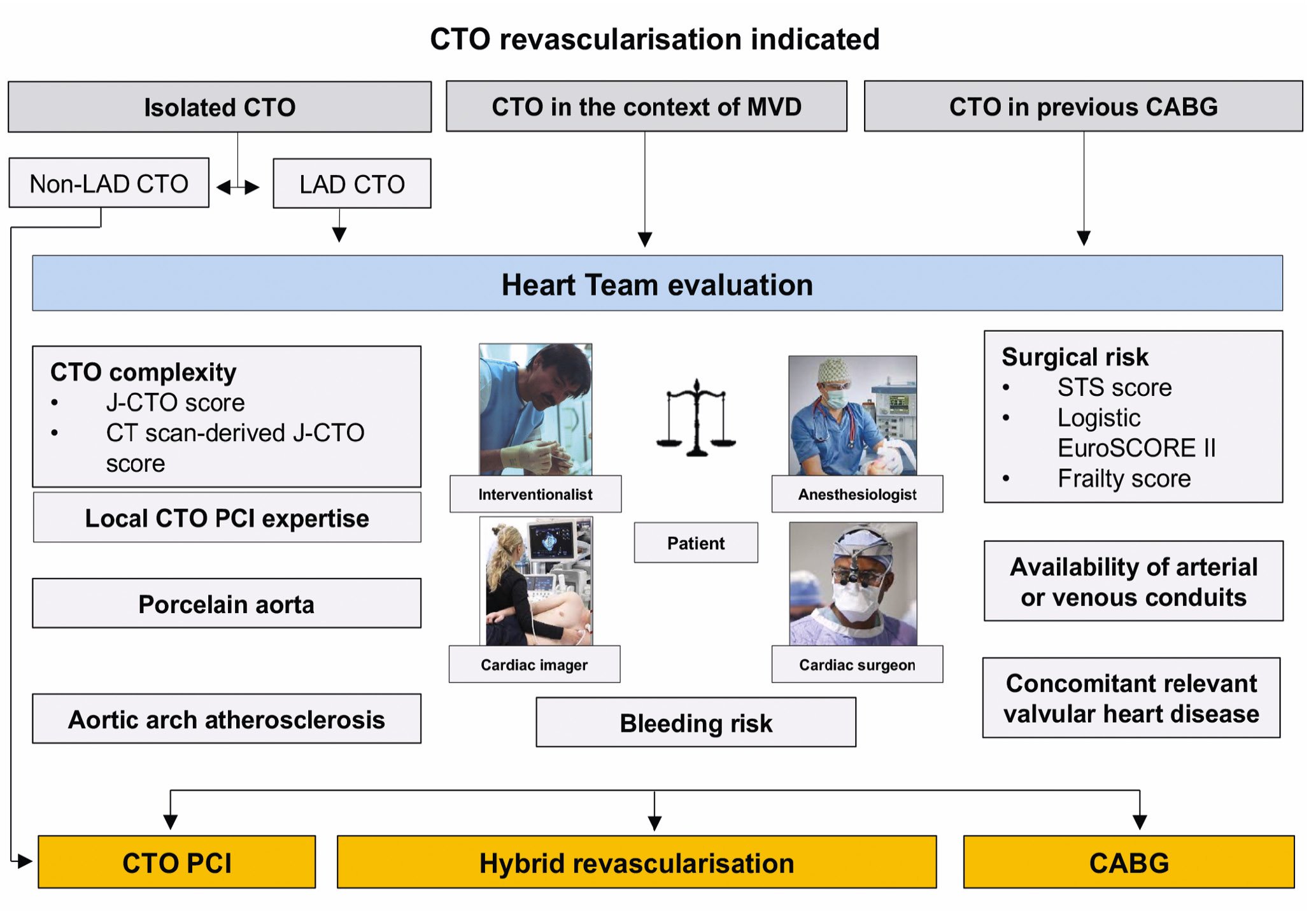
Figure 2. Definition of CTO revascularisation. Except for cases of isolated non-LAD CTO, the Heart Team must endorse the most appropriate revascularisation modality. CABG: coronary artery bypass grafting; CT: computed tomography; CTO: chronic total occlusion; EuroSCORE: European System for Cardiac Operative Risk Evaluation; J-CTO: Japanese Multicenter CTO Registry; LAD: left anterior descending artery; MVD: multivessel disease; PCI: percutaneous coronary intervention; STS: Society of Thoracic Surgeons
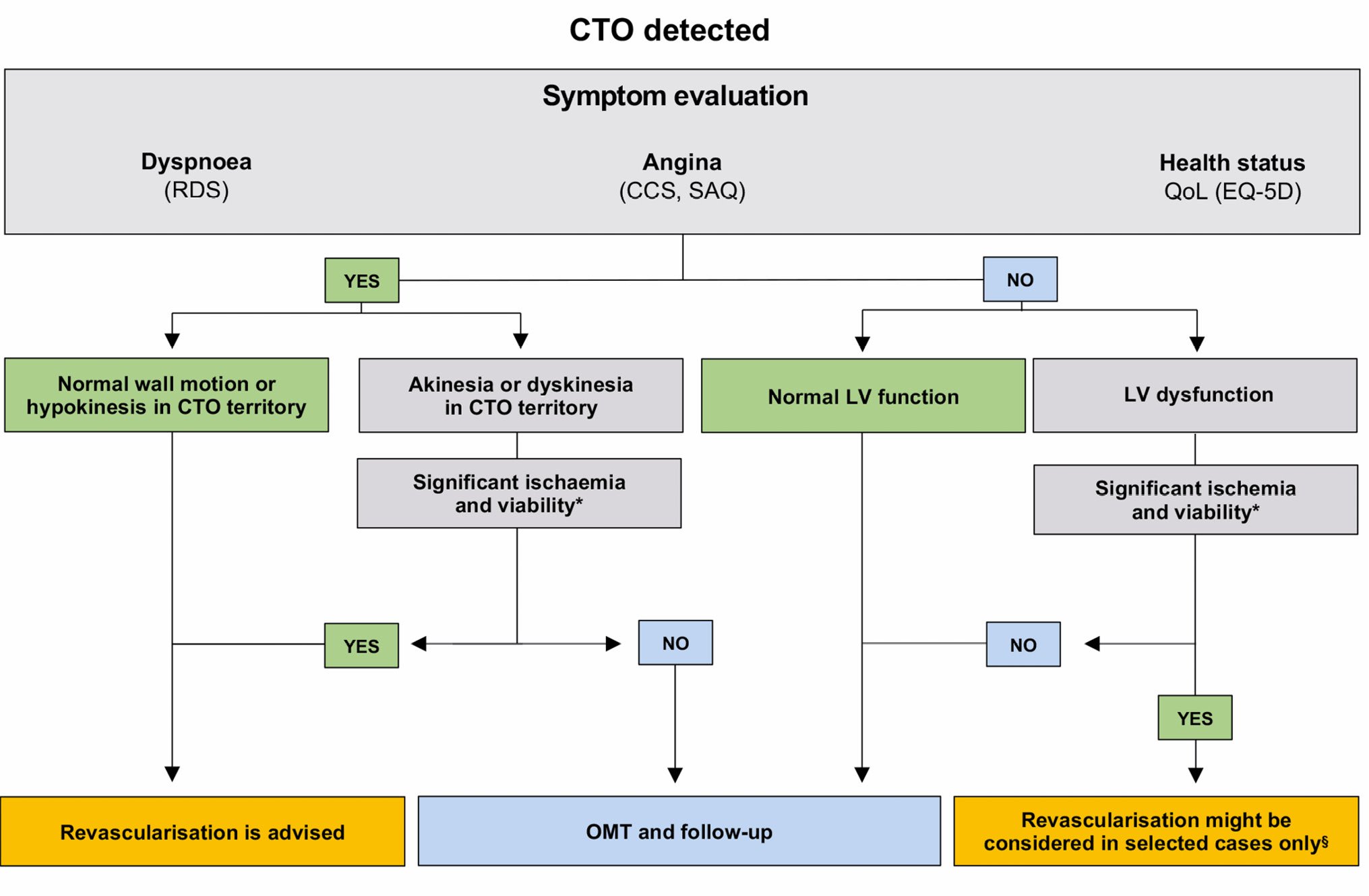
Central illustration. Flowchart of patients with CTO. *see Table 2 for inducible ischaemia/viability cutoff of each imaging modality. § E.g., young patients, with proximal LAD CTO, and demonstration of significant silent inducible ischaemia and viability. CCS: Canadian Cardiovascular Society; CTO: chronic total occlusion; EQ-5D: EuroQol five-dimensional; LV: left ventricular; MRI: magnetic resonance imaging; OMT: optimal medical therapy; QoL: quality of life; RDS: Rose Dyspnea Scale; SAQ: Seattle Angina Questionnaire
Imaging
A comprehensive imaging workup in patients with CTO ideally aims at defining the anatomy of the involved coronary artery, the amount of inducible ischaemia, and the viability of the myocardial segments supplied by it as a basis for decision-making and for procedural guidance, and eventually to predict the effect of CTO revascularisation on LV remodelling and/or residual ischaemia at follow-up (Table 2).
This section provides practical advice regarding the use of all these different imaging modalities, alone or in combination, each of them with their advantages and weaknesses.
Table 2. Evaluation of myocardial ischaemia and viability by different imaging modalities.
| Inducible ischaemia | Myocardial viability | Limitations | |
|---|---|---|---|
| ECHO | • Stress-induced wall motion abnormalities in >3 out of 17 segments • Stress-induced perfusion defect with contrast echo in >3 out of 17 segments |
• Low-dose dobutamine/low-load exercise echo wall motion abnormalities improvement in >3 out of 17 segments • Reversibility of rest or stress-induced perfusion defect with contrast echo in >3 out of 17 segments |
• Image quality • Reproducibility • Reduced sensitivity for viability assessment |
| CMR | • Stress-induced wall motion abnormalities in ≥3 out of 16 segments • Stress-induced perfusion defect in ≥2 out of 17 segments |
• Low-dose dobutamine/low-load exercise echo wall motion abnormalities improvement in ≥3 out of 16 segments • LGE <25-50% transmurality in >4 out of 17 segments |
• Availability • Time-consuming • Not suitable for patients with claustrophobia • Costs |
| SPECT and PET | • Stress-induced SPECT Tc-99m: >10% of global myocardium area • Pharmacologically induced [150]H20- or [13N]-ammonia PET imaging in >3 out of 17 segments |
• [18F]FDG PET resting uptake ≥50% • 201-T1 or Tc-99m SPECT resting uptake >50% |
• Ionising radiation exposure • Time-consuming |
| CTCA | • Stress-induced perfusion defect with contrast • FFR-CT <0.8 |
• Perfusion CT <75% transmurality in >3 out of 17 segments | • Ionising radiation exposure • Ventricular performance |
| CMR: cardiac magnetic resonance imaging; CT: computed tomography; CTCA: computed tomography coronary angiography; ECHO: echocardiography; FDG: fluorodeoxyglucose; FFR-CT: fractional flow reserve computed tomography; LGE: late gadolinium enhancement; PET: positron emission tomography; SPECT: single-photon emission computed tomography | |||
LV STRUCTURE AND FUNCTION
The primary goal of assessing left ventricular function is risk assessment for a planned CTO procedure because complications increase as LVEF decreases29. Echocardiography, especially if refined with myocardial strain imaging, is the first choice due to its wide availability30. Furthermore, echocardiography can rule out other possible concomitant pathologies, such as heart valve disease or aortic aneurysms, that must be considered before coronary revascularisation.
ISCHAEMIA QUANTIFICATION AND MYOCARDIAL VIABILITY
The revascularisation of a CTO should only be indicated if the myocardial segments supplied by it are viable and ischaemic upon pharmacological stress or exercise.
Nowadays, many different imaging tests can be used for these purposes, each of them with advantages and drawbacks (Table 2). However, the local availability of such technologies and the local imagers’ expertise can drive the preferential choice of one test over another.
Patients with a large area of viable and ischaemic myocardium are likely symptomatic and would likely derive benefit from CTO revascularisation; conversely, minor degrees of ischaemia commonly respond well to OMT3132. Furthermore, patients with extensive ischaemic burden reduction and no residual ischaemia after CTO PCI have lower rates of all-cause death and non-fatal myocardial infarction as compared with those with significant residual perfusion defect. Moreover, long-term cardiac symptom relief was associated with normalisation of hyperaemic myocardial blood flow (hMBF) levels after CTO PCI33. Stress cardiac magnetic resonance imaging (cMRI) perfusion, using a gadolinium-based contrast agent and pharmacological vasodilation, is currently considered state-of-the-art for detecting ischaemia and viability, providing precise information about fibrosis and scarring3435. Indeed, after successful revascularisation, myocardial contractility recovery of those segments supplied by the CTO vessel is likely if transmurality of late gadolinium enhancement (LGE) is <25%, while it is very unlikely if LGE is >75%. Unfortunately, for segments in the intermediate range, the predictive accuracy of LGE transmurality is limited; this aspect might explain why successful CTO PCI of a dysfunctional but viable myocardium does not lead to the expected left ventricular function recovery in all patients3536. Beyond cMRI, stress echocardiography is widely used for the assessment of myocardial viability and inducible ischaemia with treadmill exercise or dobutamine protocols. However, dobutamine echocardiography, besides its high specificity, showed reduced sensitivity in predicting the recovery of dysfunctional myocardium supplied by totally occluded vessels37. Finally, among nuclear tests, positron emission tomography (PET) should be the preferred technique for the myocardial viability assessment due to its higher spatial and temporal resolution, lower radiation dose, and shorter scan duration than single-photon emission computed tomography (SPECT)38.
GUIDANCE FOR PROCEDURAL PLANNING
For a definitive diagnosis of CTO, coronary angiography remains the gold standard. Thus, for guidance of procedural planning, a dual coronary angiography should be performed unless coronary collateral circulation originates exclusively from the ipsilateral vessel39. However, coronary computed tomography (CT) scans, particularly with three-dimensional reconstructions, are used more and more frequently, providing important complementary information to the conventional coronary angiography (location and extent of calcification, definition of morphology of proximal and distal CTO caps, occlusion length, CTO tortuosity) especially in very complex CTOs40. Such anatomical information may influence the PCI strategy and material selection for PCI. For example, in cases of heavily calcified lesions, the use of calcium modification treatment (e.g., rotational atherectomy or intravascular lithotripsy), as well as the vessel course in a very long CTO segment, can be anticipated. Furthermore, a computed tomography coronary angiography (CTCA)-derived score, such as the Computed Tomography Registry of Chronic Total Occlusion Revascularization (CT-RECTOR), was shown to be more accurate than the angiography-based Japanese Multicenter CTO Registry (J-CTO) score for grading CTO difficulty before PCI, as assessed by the time-efficient guidewire crossing4142.
Decision-making: the Heart Team
Both European and American guidelines recommend the concept of an interdisciplinary Heart Team involving cardiac surgeons, interventional and general cardiologists, cardiovascular (CV) imagers and anaesthesiologists for decision-making with different levels of evidence4344. Especially in those complex scenarios where multiple comorbidities, advanced patient age, left main and 3-vessel disease, including CTOs, make the choice of the best treatment option challenging, the Heart Team is of value to endorse the treatment strategy (Figure 2). Indeed, PCI has a low risk of immediate complications, while coronary artery bypass grafts (CABGs) showed improved long-term, event-free survival if an internal mammary artery graft to the left anterior descending artery is used45.
Beyond the anatomical (e.g., coronary structure, calcification, access to occluded segment, among others) and functional data (i.e., ischaemia and viability) relevant to assess the appropriateness of a CTO procedure, the patient’s clinical profile and their wishes also have to be taken into account when considering coronary revascularisation of CTOs. The patient must be made aware that they will undergo an intervention with a much longer duration and a higher complication rate (e.g., coronary perforations) than for non-CTO PCI (Table 1). Finally, the patient should be aware that while a symptomatic improvement or a reduction in antianginal drugs are both plausible, there is not sufficient evidence to suggest that the CTO revascularisation will prolong their survival or decrease the risk of major adverse CV events at follow-up (Table 1).
Clinical outcomes
Despite the high prevalence of CTO and the higher CV risk associated with it, CTO recanalisation traditionally represents only a small fraction of the overall volume of PCIs46. In the past, this was mainly due to a combination of low success rates, high incidence of complications, long procedural times, high costs and perceived lack of clinical benefit. However, in the last decade, along with an increased revascularisation success rate23, the pooled estimate of complication rates has decreased over time, now at a maximum of 3%, yet still remaining higher than that of non-CTO PCI4748.
Given this, the most important questions to be answered after PCI-based CTO revascularisation are the following:
1) Is there any improvement in angina, exercise capacity and/or quality of life?
2) Is there any reduction in major adverse cardiovascular events (MACE) at long-term follow-up?
3) What is the role of CTO revascularisation in patients with left ventricular dysfunction?
SYMPTOMS, QUALITY OF LIFE AND FUNCTIONAL STATUS
In stable coronary artery disease, the prevalence of angina, despite OMT, was approximately 20% in one of the largest contemporary European registries where anginal symptoms were associated with an increased risk of MACE, including cardiovascular death and non-fatal MI49. Furthermore, a non-negligible proportion of patients still complained of refractory angina despite multiple CABGs and/or PCIs1. Besides OMT and revascularisation of non-CTO lesions, CTO PCI represents an additional tool for symptom relief and improvement in quality of life5051.
Recently, the OPEN-CTO registry demonstrated that CTO PCI improved patients’ health status, as assessed by the SAQ and mean RDS scores at 1-month follow-up18. Furthermore, 3 out of the 4 main randomised trials available on this topic, EuroCTO52, COMET-CTO53, and IMPACTOR-CTO54, demonstrated that patients’ health status improved more significantly after CTO PCI than with OMT, while the DECISION-CTO trial51 did not. In the EuroCTO trial, patients undergoing PCI for CTO not only showed a marked improvement in the SAQ score (angina frequency and quality of life) compared to OMT (hazard ratios [HR] 5.23 and 6.62, respectively), but also a higher rate of complete freedom from angina (71.6% vs 57.8%; p<0.001) and a lower burden of antianginal drugs taken at 1-year follow-up52. Similarly, the COMET-CTO trial, at a mean follow-up of 275±88 days, showed a significant improvement of symptoms and quality of life measured by the SAQ in the CTO PCI group compared to the OMT group53. Finally, in the IMPACTOR-CTO trial, where 94 patients with isolated right coronary artery CTO were randomised, CTO PCI led to a significantly greater improvement in the 6-min walk distance and quality of life as assessed by the 36-Item Short Form Health Survey (SF-36) at 1-year follow-up, compared with OMT alone54.
In summary, evidence supporting CTO PCI as an effective tool for symptom relief (both angina and dyspnoea) and improvement in quality of life is based on several observational studies and on 4 randomised controlled trials, of which 3 are in favour of endovascular revascularisation, while one (DECISION-CTO) was neutral. Based on this, symptoms such as angina or dyspnoea, resistant to the best medical therapy tolerated by the patient, should be the main driver of CTO recanalisation (Table 1).
MACE REDUCTION AT LONG-TERM FOLLOW-UP
Different observational studies using a propensity score-matched analysis have demonstrated a lower incidence of MACE after CTO revascularisation as compared with OMT alone at long-term follow-up555657. Similarly, two large prospective registries, the Korean registry58 and the Canadian Multicenter Chronic Total Occlusion Registry59, showed a significant clinical benefit of CTO revascularisation over OMT alone at very long-term follow-up. The first is a single-centre, propensity-matched cohort of 1,547 consecutive patients with CTO who underwent either PCI or routine OMT. At 10 years, a significant mortality benefit was shown in the CTO PCI group as compared with OMT (13.6% vs 20.8%, HR 0.64; p=0.01)58. Similarly, in the Canadian Registry, which enrolled 1,624 patients, CTO revascularisation was associated with a lower 10-year incidence of MACE, including all-cause mortality (22.7% vs 36.6%), future revascularisation (14.0% vs 22.8%), and ACS hospitalisation (10.0% vs 16.6%) as compared with OMT59. Similarly, at 3-year follow-up, the EuroCTO trial confirmed no differences in the rates of cardiovascular death or myocardial infarction between PCI or OMT among patients with a remaining single coronary CTO, but the MACE rate was higher in the OMT group due to more ischaemia-driven revascularisations60.
Conversely, the two other randomised trials, DECISION-CTO51 and EXPLORE61, did not confirm a long-term MACE reduction with CTO revascularisation as compared with OMT. However, many caveats exist for both trials, which have been described in detail previously62.
Therefore, although randomised controlled trials provide the highest level of evidence in the currently used guidelines, they also have some limitations and give conflicting results. Moreover, propensity-matched analysis studies and registries are prone to undetected bias and often have a lack of an independent MACE adjudication. As such, registries can only be considered hypothesis-generating. Furthermore, CTO revascularisation compared with OMT now faces fierce competition by effective anti-ischaemic, antithrombotic and hypolipidaemic remedies (Table 1). Nevertheless, further large-scale, randomised trials, with long-term follow-up, including patients with depressed LVEF, comorbidities and significant symptoms, are needed to determine whether CTO revascularisation is indeed superior to OMT in terms of long-term MACE reduction.
CTO REVASCULARISATION OF PATIENTS WITH REDUCED LEFT VENTRICULAR EJECTION FRACTION
Although some previous studies have shown LVEF recovery after successful revascularisation of the CTO territory, especially among patients with more severe LV dysfunction, significant myocardial inducible perfusion defect and viability, these data were not confirmed in the randomised REVASC trial24253263. In this study, the mean baseline LVEF was only mildly/moderately reduced in both the OMT and CTO PCI groups (59.6 [45.8 to 64.3] and 54.7 [42.9 to 65.1], respectively), with one possible explanation for this being the neutral effect of coronary revascularisation on LV recovery (Table 1).
Similarly, the relationship between LVEF recovery and long-term patient outcomes is still debated (Table 1). In the REVASC trial, the CTO PCI group showed a higher MACE rate reduction, as compared with the group managed by OMT, driven mostly by lower repeated intervention rate at 1-year follow-up (16.3% vs 5.9%; p=0.02)63.
Furthermore, Schumacher et al have recently demonstrated that while extensive ischaemia reduction after CTO PCI led to significantly better survival, free of death and MI, among patients with LV dysfunction, this was not the case among patients with preserved LV function64.
Of course, the present study is not randomised and should be considered as hypothesis-generating. Therefore, despite successful CTO revascularisation of dysfunctional myocardium not being systematically followed by LVEF recovery, future large randomised studies are warranted to conclusively define whether CTO revascularisation of patients with severe LV dysfunction may be beneficial at long-term follow-up. Indeed, this population is not adequately represented in the current studies.
Conclusions
Percutaneous CTO interventions have reached a high level of success with acceptable complication rates when in the hands of expert operators. Symptom improvement, on top of medical therapy, is the main goal of CTO PCI, while MACE reduction at follow-up remains uncertain, as the currently available trials were underpowered and led to conflicting results. A rational patient selection, based on clinical symptoms, different imaging modalities and a multidisciplinary approach, is key for a successful CTO revascularisation. Finally, in situations where multiple comorbidities make the choice of the best treatment option challenging, the Heart Team is essential to endorse the treatment strategy.
Conflict of interest statement
N. Bonaros: Medtronic and Edwards Lifesciences; B. Cosyns: research funding from Abbott; C. Di Mario: Boston Scientific, Medtronic, Behring, Idorsia, Philips, Shockwave Medical, Edwards Lifesciences; D. Z. Dudek: Abbott, Balton, Mac's Medical, Chemie AG, Ferrer Internacional, Aspironix, Abiomed, Hemolens, AstraZeneca, Biotronik, Boston Scientific, Bracco Pharmaceutical, Edwards Lifesciences, GE HealthCare, Medtronic, Procardia, B. Braun, Teleflex, Sanofi Aventis, Philips, SMT, Gedeon Richter, Cardinalhealth, Siemens Healthcare, Terumo Inc; B.L.M. Gerber: Pfizer, Daiichi Sankyo, Bristol-Myers Squibb, Sanofi Aventis; J. Hill: Abbott Vascular, Shockwave Medical, Neovasc, Boston Scientific, Abiomed; T.F. Lüscher: Acthera, Daiichi Sankyo, Amgen, Novartis, Boehringer-Ingelheim, Philips, e-therapeutics, Open Health, Novo Nordisk, AstraZeneca, Vifor International, Abbott; M. McEntegart: Boston Scientific, Teleflex, Shockwave Medical, Medtronic, Biosensors; B. Vaquerizo Montilla: Boston Scientific, Medtronic, Lifetech; G.S. Werner: Abbott Vascular, Daiichi Sankyo, Shockwave Medical, Terumo Inc, Siemens Healthcare, Philips, Orbus, Asahi. The other authors have no conflicts of interest to declare.

