
Abstract
Aims: We sought to investigate the incidence, predictors, and clinical outcomes of stent optimisation with intravascular ultrasound (IVUS) in long coronary lesions treated with new-generation drug-eluting stents (DESs).
Methods and results: From four randomised trials comparing IVUS and angiography guidance in long (≥26 mm) or chronic total occlusion coronary lesions, a total of 1,396 patients who underwent IVUS-guided intervention were classified into two groups (stent optimisation and non-optimisation) according to optimisation criteria (minimal stent area [MSA] ≥5.5 mm2 or 80% of mean reference lumen area [MLA]). Major adverse cardiac event (MACE) occurrence, defined as a composite of cardiac death, myocardial infarction, stent thrombosis, or target vessel revascularisation, was compared. Stent optimisation was not met in 578 (41%) patients. Predictors of non-optimisation were older age, longer lesion length, and smaller stent diameter. The MACE rate was significantly higher in the non-optimisation versus the stent optimisation group (4.8% vs 1.9%, log-rank p=0.002; adjusted hazard ratio 2.95, 95% CI: 1.43-6.06). Among possible combinations of absolute and relative expansion criteria, the one best predicting MACE was at least one of MSA ≥5.4 mm2 and/or ≥80% of MLA (Youden index=0.264).
Conclusions: Achieving stent optimisation using IVUS evaluation was associated with favourable outcomes in IVUS-guided, new-generation DES implantation for long coronary lesions including CTOs.
Introduction
In the era of drug-eluting stents (DESs), there is growing evidence from randomised controlled trials and meta-analyses that intravascular ultrasound (IVUS)-guided percutaneous coronary intervention (PCI) compared to conventional angiography guidance could improve clinical outcomes1,2,3,4,5,6,7,8. For IVUS-guided PCI, authors’ own criteria of stent optimisation were mainly based on the various degrees of stent expansion4,5,8,9,10,11. However, in previous studies achieving favourable clinical outcomes with IVUS guidance, a considerable number of patients did not meet the predefined stent optimisation targets. In addition, the clinical implications of non-optimisation have not been fully elucidated in long lesions12.
Therefore, we conducted a comprehensive analysis of individual patient-level data from randomised trials targeting long or chronic total occlusion (CTO) lesions to evaluate the incidence, predictors, and clinical outcomes of stent optimisation following new-generation DES implantation. We also investigated the association between the absolute and relative stent expansion and determined the optimal combination criteria of absolute and relative expansion predicting adverse outcomes.
Methods
STUDY DESIGN AND POPULATION
This study included four randomised controlled trials comparing IVUS and angiography guidance for long or CTO lesions treated by new-generation DESs, with available patient-level data for pooled analysis: RESET (Real Safety and Efficacy of a 3-Month Dual Antiplatelet Therapy Following Zotarolimus-Eluting Stents Implantation), CTO-IVUS (Impact of IVUS-guided Chronic Total Occlusion InterVention With DrUg-eluting Stents on Mid-term Angiographic and Clinical Outcomes), IVUS-XPL (Impact of IntraVascular UltraSound Guidance on Outcomes of Xience Prime Stents in Long Lesions), and ULTRA-ZET (Intravascular ULTrasound Guided Versus Conventional Angiography Guided Strategy to Deploy Zotarolimus and Everolimus Eluting Third Generation Stents in the Long Coronary Artery Lesions) (Figure 1). Detailed explanations of these studies are provided in Supplementary Table 1,2,4,5. Briefly, we included the studies which enrolled patients with long lesions requiring a stent length ≥26 mm or CTO. The statisticians extracted patient-level data from each trial by direct access to the study databases. Data on baseline patient characteristics, procedure information, and clinical events were collected. These patient data were pooled and analysed in a single data set.
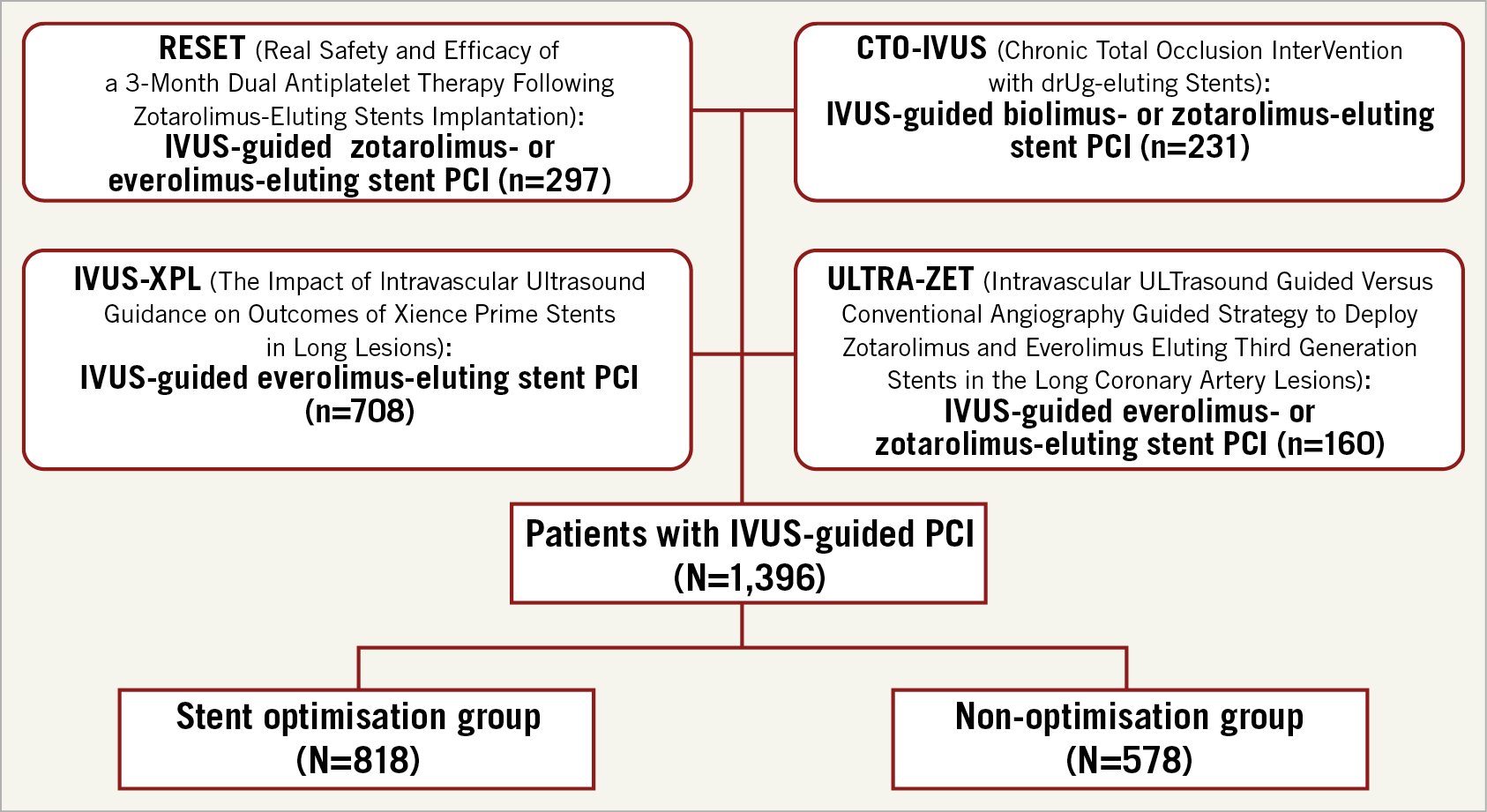
Figure 1. Study flow. IVUS: intravascular ultrasound; PCI: percutaneous coronary intervention
IVUS EXAMINATIONS AND ANALYSES
IVUS examinations were performed with commercially available imaging systems (40 MHz IVUS catheter [Boston Scientific, Marlborough, MA, USA]; 20 MHz IVUS catheter [Volcano Corp, Rancho Cordova, CA, USA]). All images were analysed at the Cardiovascular Research Center core laboratory (Seoul, South Korea) by analysts blinded to patient and procedural information. Detailed explanations regarding imaging acquisition were provided in previous studies2,4,5. Using planimetry software (echoPlaque™ 3.0; INDEC Systems, Santa Clara, CA, USA), cross-sectional lumen, stent, and vessel areas were measured at proximal and distal references (within 10 mm of the proximal or distal stent edge) and the minimal stent area (MSA) site according to current guidelines13.
Stent expansion was classified as absolute expansion, defined as MSA with an absolute measure14,15,16, and relative expansion, defined as the percent ratio of MSA to mean reference lumen area (MLA)4,5,9,10. In this study, the main criteria of stent optimisation were defined as MSA ≥5.5 mm2 or 80% of MLA according to the most recent expert consensus document12. Depending on whether the stent optimisation criteria were met or not, all enrolled patients were classified into either the stent optimisation or the non-optimisation group.
ENDPOINTS, DEFINITIONS, AND FOLLOW-UP
The primary endpoint in this study was the occurrence of major adverse cardiac events (MACE), defined as a composite of cardiac death, myocardial infarction (MI), stent thrombosis (ST), or target vessel revascularisation (TVR). The secondary endpoints were: 1) individual components of the primary endpoint; 2) incidence of stent optimisation; 3) major predictors for non-optimisation; and 4) the optimal combination criteria of the absolute and relative expansion for predicting MACE. Detailed definitions of clinical endpoints are presented inSupplementary Appendix 1. Clinical follow-up and assessment were performed in the hospital after 1, 3, 6, and 12 months either by clinic visit or by telephone interview.
STATISTICAL ANALYSIS
Continuous variables are presented as mean±standard deviation, and categorical variables are presented as numbers (percentages). Continuous and categorical variable data were analysed using Student’s t-tests and chi-square tests. Cumulative incidence values were calculated using the Kaplan-Meier method and compared using log-rank tests. Logistic regression analysis was performed to identify predictors of non-optimisation. Any variables with p<0.1 on univariate analysis were included in the multivariate logistic regression analysis. To estimate the effect of stent optimisation on clinical outcomes, hazard ratios (HRs) were calculated using the Cox proportional hazards model. In multivariate Cox regression analysis, HRs were adjusted for age, sex, hypertension, diabetes mellitus, current smoking status, prior MI, prior PCI, prior coronary artery bypass graft surgery, clinical presentation, ejection fraction, treated vessels, CTO, DES types, stented length, number of stents per lesion, maximum stent diameter, high-pressure post-dilation, and preprocedural quantitative coronary angiography (QCA) parameters including reference lumen diameter and minimal lumen diameter. The analysis was performed using per-protocol analysis. The subgroup analysis was performed according to baseline characteristics. Receiver operating characteristic (ROC) analysis was performed to determine the best cut-off values for predictors of non-optimisation and expansion criteria predicting MACE. A simple linear regression analysis was performed to evaluate the association between the absolute (MSA) and the relative stent expansion (the ratio of MSA to MLA). To compare the performance of optimisation criteria to predict MACE occurrence, the Youden index (sensitivity+specificity-1) was calculated. Two-sided p-values were used, and p<0.05 was considered statistically significant. All analyses were performed using R version 3.5.2 (R Foundation for Statistical Computing, Vienna, Austria).
Results
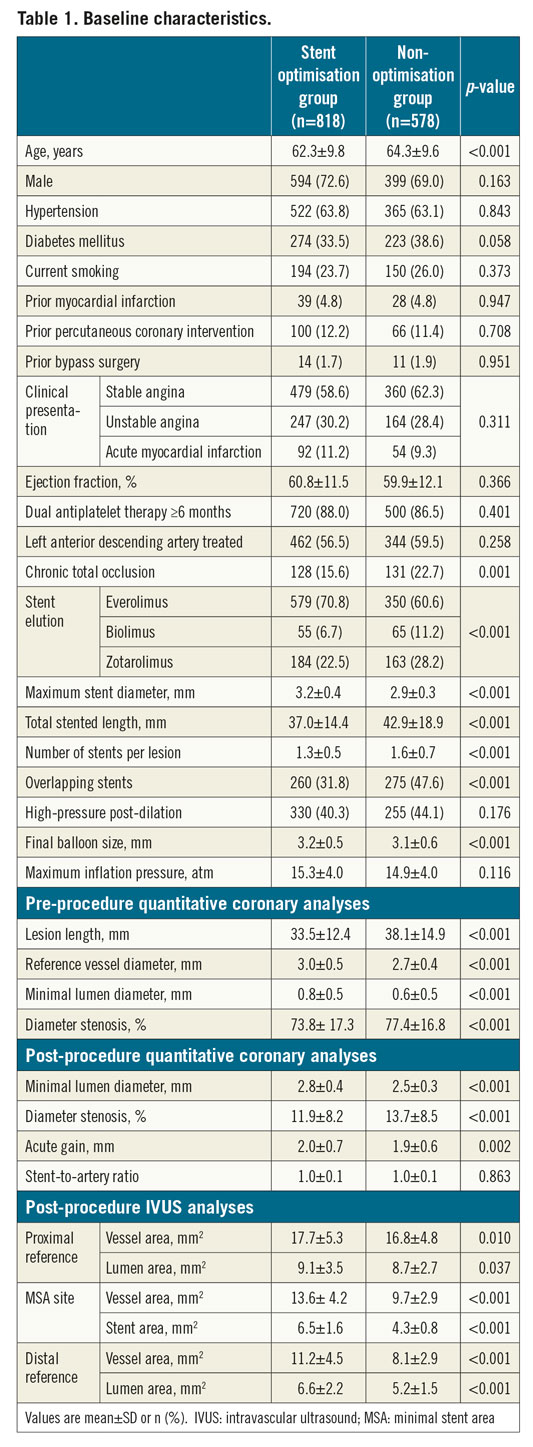
In four randomised trials targeting long or CTO lesions, a total of 1,396 patients underwent IVUS-guided new-generation DES implantation (Figure 1). Of these cases, stent optimisation criteria were met in 818 (58.6%) patients (stent optimisation group) and were not met in 578 (41.4%) (non-optimisation group). The baseline characteristics of both groups are presented in Table 1. Compared to patients in the stent optimisation group, patients in the non-optimisation group were more likely to be older, have different types of DES implanted with more CTO lesions, smaller stent diameters, longer stent lengths, overlapping stents, multiple stents per lesion, and smaller balloon sizes used. QCA analyses revealed that the non-optimisation group had a longer lesion length, smaller reference vessel diameter, preprocedural and post-procedural minimal lumen diameter, and acute gain compared to the stent optimisation group.
In IVUS analyses, vessel or lumen areas at proximal and distal reference segments were smaller in the non-optimisation group. MSA was significantly smaller in the non-optimisation group than in the stent optimisation group (Table 1).
PREDICTORS OF NON-OPTIMISATION FOR IVUS CRITERIA
By multivariate logistic regression analysis, older age, longer lesion length, and smaller maximum stent diameter were independently associated with non-optimisation (Supplementary Table 2). The best cut-off values predicting non-optimisation after DES implantation were age ≥72 years, lesion length ≥39 mm, and maximum stent diameter <3.0 mm (Figure 2A-Figure 2C). When analysing the incidences of non-optimisation based on these cut-off values, the rates were significantly different among various subgroups (Figure 2D).
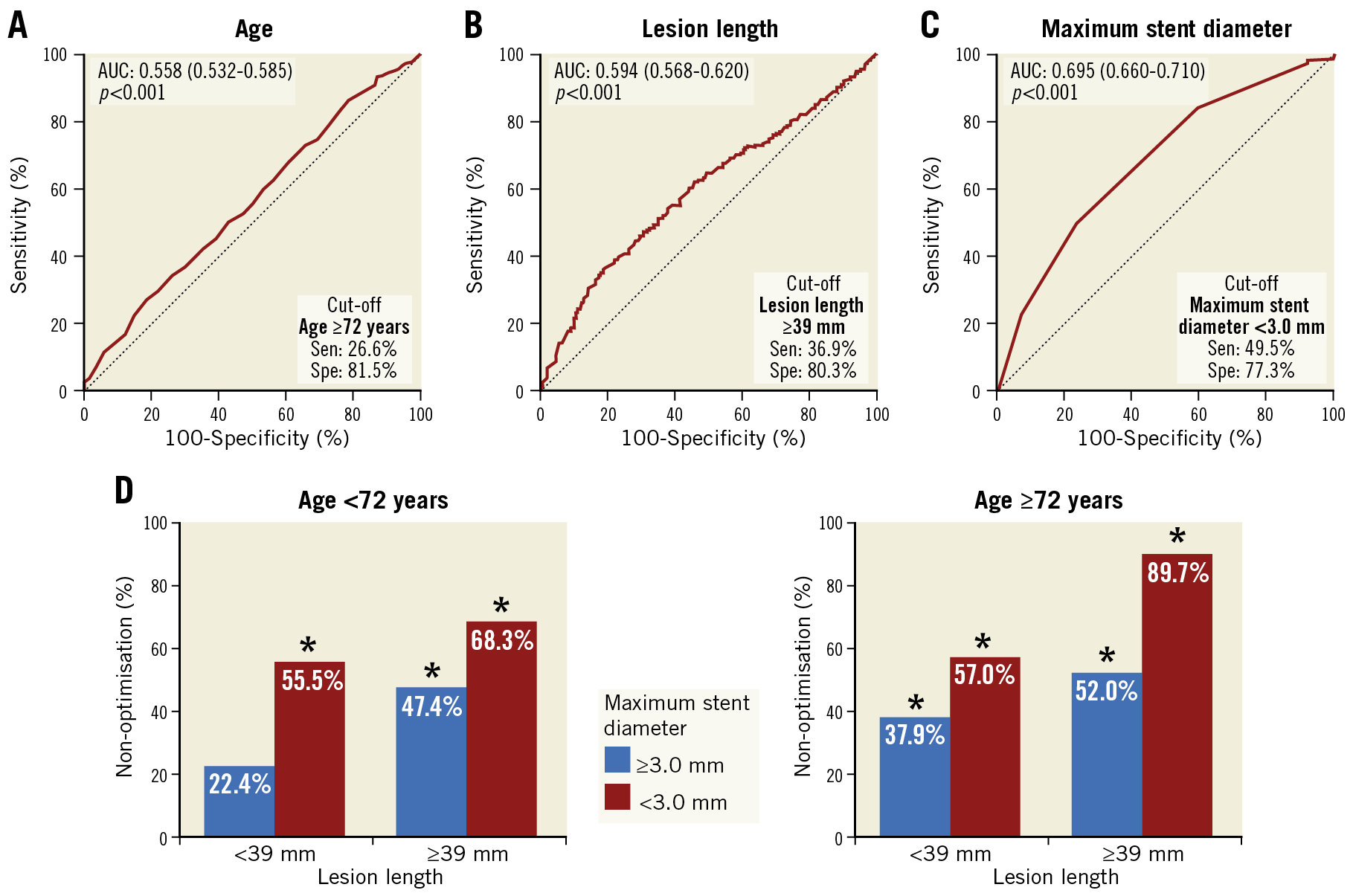
Figure 2. Predictors of non-optimisation for IVUS criteria. A) – C) Receiver operating characteristic curves of predictors for non-optimisation showing the capacities and optimal cut-off values. D) Non-optimisation incidences by subgroup according to the predictors. *p<0.001 compared to the patients without any non-optimisation predictors. AUC: area under the curve
CLINICAL OUTCOMES WITH FULFILMENT OF IVUS OPTIMISATION CRITERIA
The analyses regarding clinical outcomes of both groups are presented in Figure 3 and Table 2. MACE occurred in 27 (4.8%) patients in the non-optimisation group and 15 (1.9%) patients in the stent optimisation group (log-rank p=0.002; unadjusted HR 2.58, 95% confidence interval [CI]: 1.37-4.84) (Figure 3A). The non-optimisation group also had significantly higher rates of a fatal composite event including cardiac death, MI, and ST (p=0.017) (Figure 3B), ST (p=0.039) (Figure 3E), and TVR (p=0.006) (Figure 3F). In multivariate Cox regression analysis, non-optimisation was associated with increased risk of MACE (adjusted HR 2.95, 95% CI: 1.43-6.06), the fatal composite event, and TVR (Table 2). No significant interactions were observed in the post hoc subgroup analysis; the effects of non-stent-optimisation on the occurrence of MACE were consistent across various subgroups (Supplementary Figure 1).
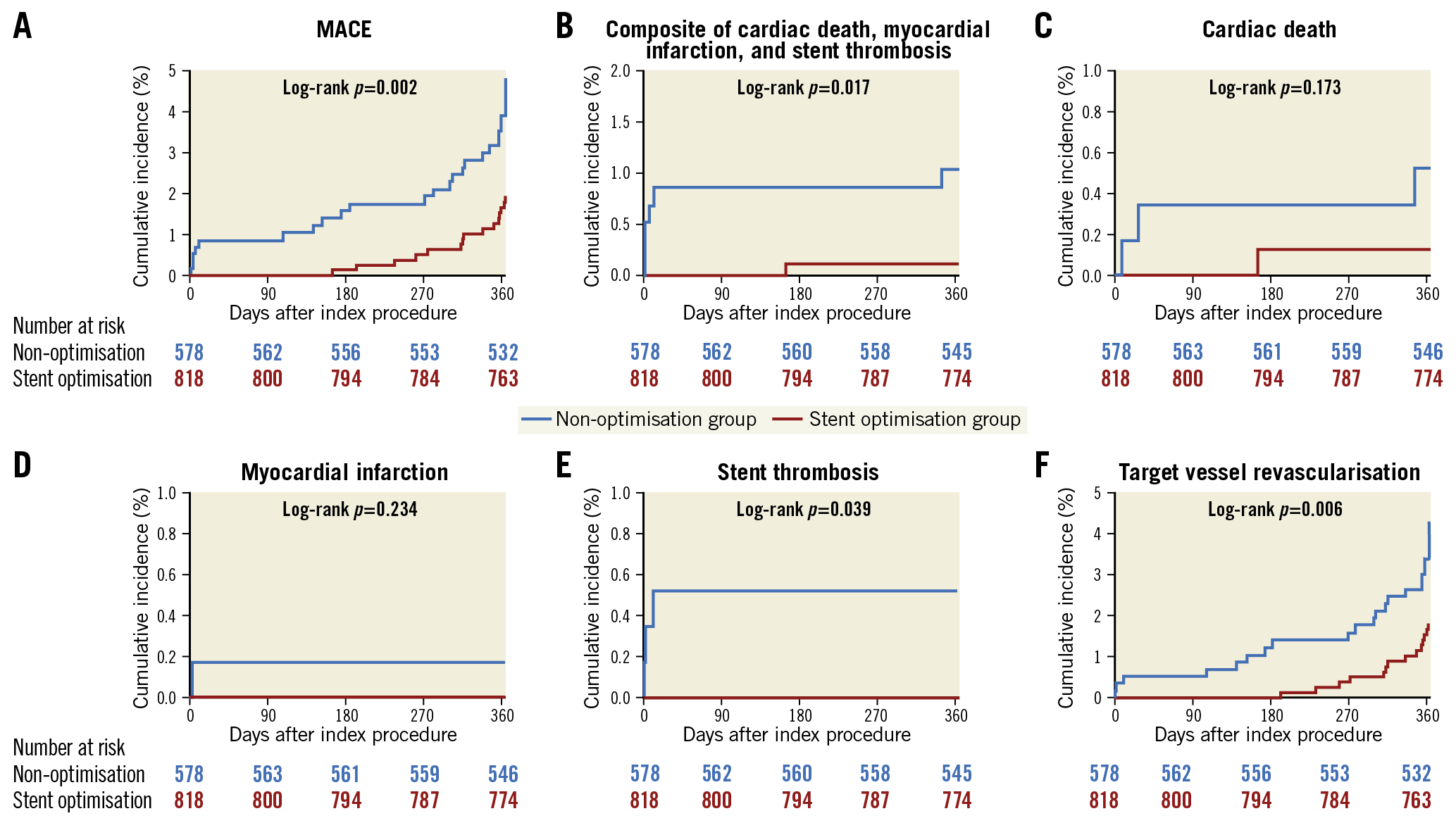
Figure 3. Kaplan-Meier estimates of clinical outcomes for the stent optimisation and non-optimisation groups. MACE: major adverse cardiac events
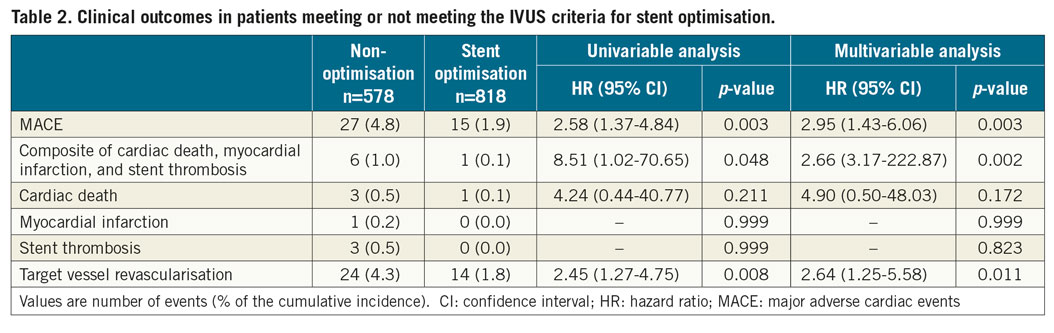
When comparing the cumulative incidence of MACE among the groups according to whether meeting each individual absolute (MSA ≥5.5 mm2) and relative expansion (MSA ≥80% of MLA) criterion, the MACE rate was significantly higher in the patients who met neither absolute nor relative expansion criteria (MSA <5.5 mm2 and <80% of MLA) than in those meeting at least one of the absolute or relative expansion criteria (all log-rank p<0.05), without significant differences among the patients meeting only one or both (Figure 4).
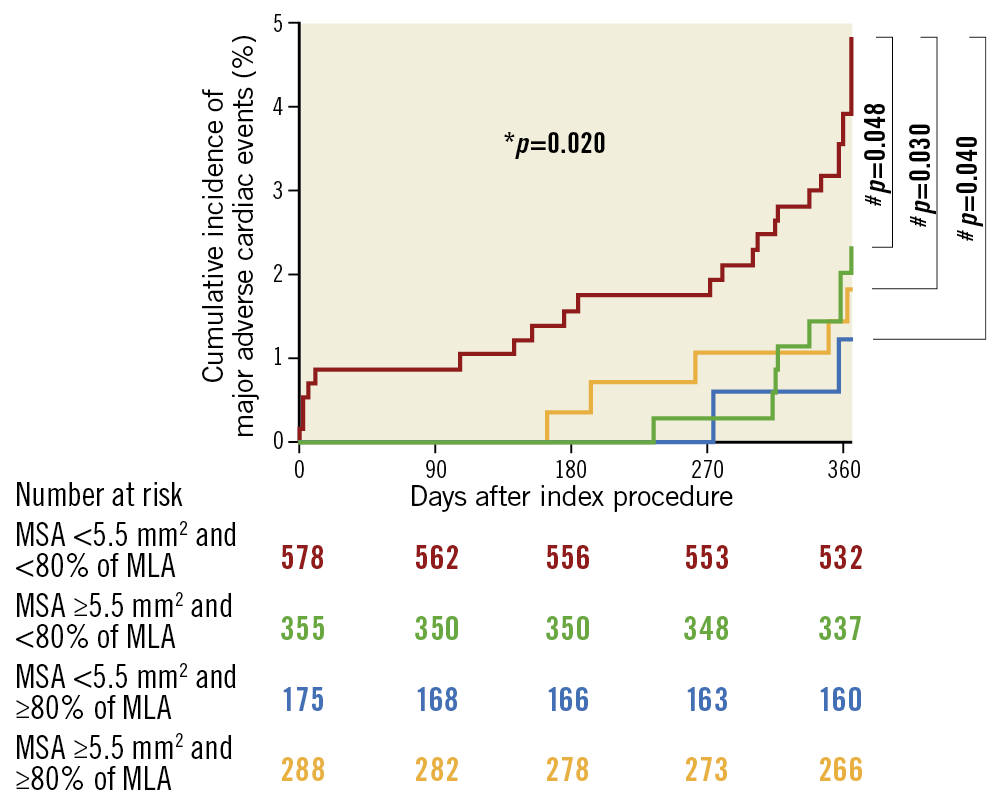
Figure 4. Kaplan-Meier estimates of major adverse cardiac events according to whether meeting either the absolute or relative expansion criteria. * Comparison among four groups by the log-rank test. #Comparison between two groups by the log-rank test. MLA: mean reference lumen area; MSA: minimal stent area
ASSOCIATION BETWEEN THE ABSOLUTE AND RELATIVE EXPANSION AND THE BEST PREDICTIVE COMBINATION CRITERIA
The absolute value of MSA was significantly correlated with the ratio of MSA to MLA (p<0.001) (Figure 5A). The correlation was stronger in those with longer lesion length (≥34.5 mm, the median lesion length) than in those with lesion length <34.5 mm (R2=0.226 vs 0.134, p=0.012) (Supplementary Figure 2). The ROC curves of absolute and relative expansion for predicting MACE are presented in Figure 5B. There was no significant difference of the area under the curve (AUC) between absolute and relative expansion (DeLong test p=0.531): AUCs of absolute MSA and MSA to MLA ratio were 0.605 (95% CI: 0.501-0.710) and 0.642 (95% CI: 0.540-0.745). The optimal cut-off values predicting MACE were 5.6 mm2 for MSA and 70% for the MSA to MLA ratio, respectively. When using the combined criteria using these optimal cut-offs, the MACE rate was significantly higher in the non-optimisation group than in the stent optimisation group (log-rank p=0.003; adjusted HR 2.45, 95% CI: 1.34-4.50) (Figure 5C).
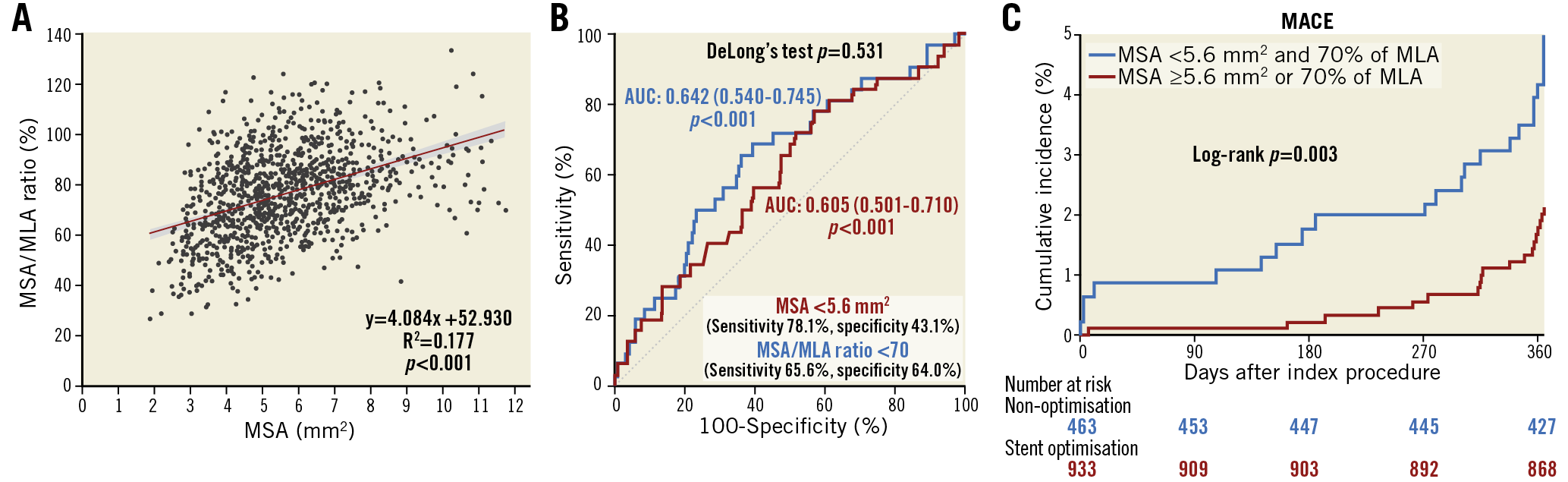
Figure 5. Association between the absolute and relative stent expansion and the best predictive expansion criteria. A) Association between absolute and relative stent expansion. B) Receiver operating characteristic curves of absolute and relative expansion showing the capacities and optimal thresholds for predicting major adverse cardiac events. C) Kaplan-Meier estimates of major adverse cardiac events for the stent optimisation and non-optimisation groups by applying the optimal cut-off values of absolute and relative expansion. AUC: area under curve; MACE: major adverse cardiac events; MLA: mean reference lumen area; MSA: minimal stent area
The comparison of Youden indices for determining the optimal combination of absolute and relative expansion criteria predicting the occurrence of MACE is presented in Figure 6.
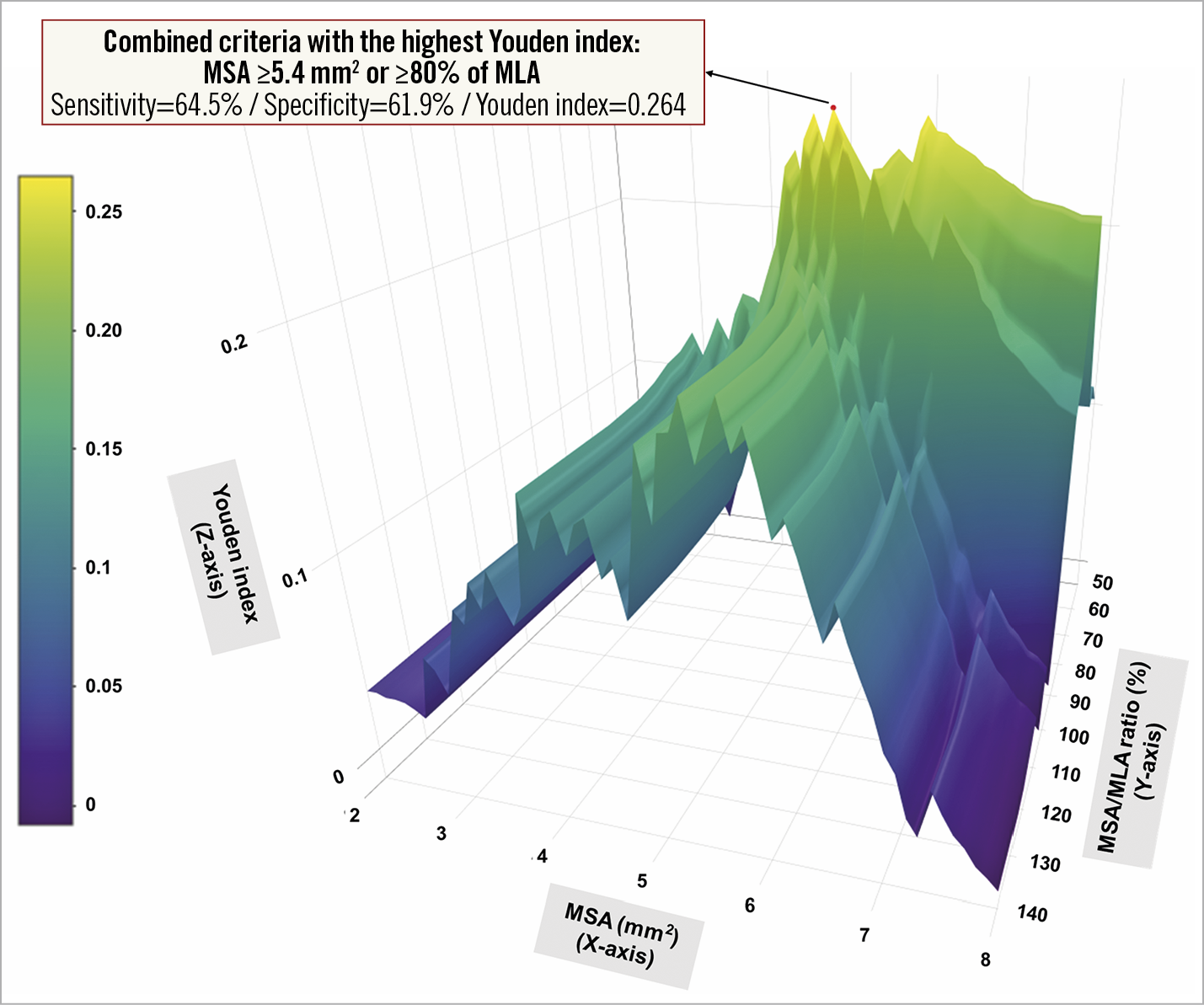
Figure 6. Comparison of predictive abilities for the occurrence of major adverse cardiac events among possible combinations of absolute and relative expansion criteria. To compare the performance to predict MACE occurrence, the Youden index (sensitivity + specificity – 1, Z-axis) was calculated for each combination of absolute (MSA ≥2-8 mm, X-axis) and relative expansion (MSA/MLA ratio ≥50-140%, Y-axis) criteria.
Among the possible combinations, at least one of MSA ≥5.4 mm2 and/or ≥80% of MLA was best predictive with the greatest Youden index of 0.264 (sensitivity=64.5% and specificity=61.9%). When using this combination, the non-optimisation group showed a significantly higher MACE rate compared to the stent optimisation group (4.8% vs 2.0%, log-rank p=0.003; adjusted HR 2.65, 95% CI: 1.29-5.44).
Discussion
There were four principal findings from our comprehensive analyses of individual patient-level data from four randomised trials targeting long or CTO lesions: 1) under IVUS guidance, 41.4% did not meet IVUS criteria for stent optimisation following new-generation DES implantation; 2) predictors of non-optimisation were older age (≥72 years), longer lesion length (≥39 mm), and smaller stent diameter (<3.0 mm); 3) the non-optimisation group showed significantly higher rates of MACE or fatal events including cardiac death, MI, or ST (in particular, compared to the patients fulfilling either absolute or relative expansion, the patients who met neither of them had the worst clinical outcomes); 4) absolute and relative stent expansion showed a statistically significant correlation and both could predict the occurrence of MACE. The best predictive combination criteria were MSA ≥5.4 mm2 and/or ≥80% of MLA, nearly identical to those in the recent expert consensus document12.
A considerable proportion of patients with IVUS guidance did not meet the IVUS criteria for stent optimisation; the implications of incidence, predictors, and outcomes had not been fully analysed for this group. In this study targeting long or CTO lesions following new-generation DES implantation, over one third of patients did not meet the IVUS criteria for stent optimisation. Although caution is needed in interpreting our results due to a heterogeneity of optimisation criteria across the four included studies, the following factors significantly affected stent optimisation - age, lesion length, and stent diameter. Older age is independently associated with coronary artery calcification, which could have a negative effect on stent expansion17,18. Longer lesion length and smaller stent diameter are well-known independent risk factors for stent failure including restenosis, which may be associated with stent underexpansion14,19,20. The incidence of non-optimisation in patients with triple predictors of non-optimisation (age ≥72 years, lesion length ≥39 mm, or stent diameter <3.0 mm) was about 90% (89.7%) in this study. More careful IVUS assessment and a more careful strategy are necessary for such patients with multiple predictors for non-optimisation after DES implantation. Studies on how to achieve stent optimisation and specific optimisation criteria for these high-risk patients and lesions are needed.
Regarding clinical outcomes, the patients who did not meet the criteria for stent optimisation showed significantly worse clinical outcomes than those who did meet the IVUS criteria in long coronary lesions, even with IVUS guidance using new-generation DES implantation. In each included study, the between-group difference in MACE was not statistically different due to the relatively low event rate, even though all four trials showed a trend in favour of stent optimisation (Supplementary Figure 1). In this pooled analysis, both the hard clinical endpoints, including the composite of cardiac death, MI, or ST, and the efficacy endpoints, such as TVR, were significantly higher in the patients who did not meet the optimisation criteria. These clinical benefits could be attributable to the increased power according to the use of the individual-level data from 1,396 patients. Improving clinical outcomes and maximising the impact of IVUS guidance will require meticulous IVUS evaluations and assessments before and after stenting; these would require going beyond simply performing IVUS catheter crossing and achieving visual confirmation.
As stent underexpansion is a major predictor of stent failure14, its evaluation is the most important component of the IVUS criteria for stent optimisation. With respect to absolute expansion, achieving a greater MSA has been associated with better stent patency and a lower risk of TVR, especially after DES introduction14,15,16. Sonoda et al reported that the optimal MSA threshold predicting long-term stent patency was <5.0 mm2 for sirolimus-eluting stents15. Hong et al suggested an MSA of 5.5 mm2 as the cut-off best discriminating subsequent events in IVUS-guided sirolimus-eluting stent implantation for non-left main lesions14. In addition, other DES studies using different stent types reported similar definite values as significant factors for predicting stent failure16. Thus, previous studies proposed various IVUS criteria for stent optimisation with respect to either absolute expansion (MSA ≥5 or 5.5 mm2) or relative expansion (MSA ≥80 or 90% of MLA)4,5,9,10,11,14,15,16. Several different criteria based on these findings have been employed in different clinical studies evaluating the effect of IVUS on clinical outcomes4,5,9,11. However, optimisation criteria based on absolute cut-off values might vary depending on vessel size and result in relative stent undersizing and oversizing in large and small vessels, respectively12. Particularly in small vessels, the attainment of MSA ≥5 or 5.5 mm2 might not be easy and could cause complications, such as perforation or edge dissection, by the vigorous post-dilation. Therefore, the criteria for optimal stent expansion for small and long lesions can be different from those for larger vessels. In the present study, the relative expansion significantly correlated with the absolute expansion (as the lesion was longer, the correlation got stronger) and was a statistically significant predictor for the occurrence of MACE. When analysing the risk of MACE according to whether meeting absolute or relative expansion criteria, meeting only one of the absolute or relative expansion criteria showed low MACE rates, comparable to those of meeting both of them, whereas meeting neither absolute nor relative expansion had the worst outcomes. In the decision on IVUS-optimised criteria for long coronary artery stenoses treated by new-generation DES, relative stent expansion was useful for determining stent optimisation, and the evaluation of stent optimisation by using the combination of absolute and relative expansion criteria (meeting either relative or absolute criteria) would be more suitable, practical, and predictive. The best predictive combination in this study was at least one of MSA ≥5.4 mm2 and/or ≥80% of MLA, which was almost the same with MSA ≥5.5 mm2 and/or ≥80% of MLA, as suggested in the most recent expert consensus document and mainly analysed in this study12.
Limitations
The limitations of this study are as follows. First, although we evaluated optimisation criteria for long coronary lesions, including CTO, the criteria of the four enrolled studies were not identical. In addition, CTOs are usually long lesions but can sometimes have non-diffuse long features. Thus, general extension of our results to entire long lesions might require care. Second, analyses of qualitative IVUS assessment, including calcification, were not performed. Volumetric assessment was also not performed. Third, optimisation criteria usually include the status of stent apposition and post-stenting edge dissection; however, these were not assessed in this study. Finally, a one-year follow-up period may not be sufficient for assessing long-term clinical outcomes.
Conclusions
Achieving stent optimisation with IVUS evaluation was associated with favourable outcomes in IVUS-guided, new-generation DES implantation for long coronary lesions including CTO. This study confirmed that the combination of absolute and relative stent expansion criteria was useful and the optimisation criteria (of at least one of MSA ≥5.5 mm2 and/or ≥80% of MLA) according to the recent expert consensus document were predictive for the occurrence of MACE in long coronary lesions.
|
Impact on daily practice In previous studies achieving favourable clinical outcomes with IVUS guidance, a considerable number of patients did not meet the predefined stent optimisation targets, reflecting the difficulty in sufficiently meeting IVUS criteria for stent optimisation, particularly for long coronary lesions even with the use of IVUS during procedures. In our comprehensive analysis of individual patient-level randomised trials targeting long or chronic total occlusion lesions, achieving stent optimisation on IVUS evaluation was strongly associated with favourable outcomes. More careful IVUS assessment and a more careful strategy are necessary for patients with multiple predictors for non-optimisation (old age, longer lesion, and small stent diameter). |
Funding
This study was supported by a grant from the Korea Healthcare Technology Research & Development Project, Ministry for Health & Welfare, Republic of Korea (Nos. A085136 and HI15C1277), the Mid-Career Researcher Program through an NRF grant by the MEST, Republic of Korea (No. 2015R1A2A2A01002731), and the Cardiovascular Research Center, Seoul, Republic of Korea.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

