
While coronary angiography is the gold standard for diagnostic assessment and treatment guidance of coronary artery disease, it is limited by depicting contrast-filled lumen rather than the vessel wall itself. Intravascular ultrasound (IVUS) and its light analogue, optical coherence tomography (OCT), can overcome inherent limitations of the “lumenogram” by directly visualising the coronary arteries. Intracoronary imaging guidance of percutaneous coronary interventions (PCI) not only facilitates a better procedural result but also has the potential to improve clinical outcomes, mainly by reducing the need for repeat revascularisation. Early studies in the era of bare metal stents (BMS), followed by randomised controlled trials (RCTs) using drug-eluting stents (DES), provided robust evidence of a reduction in ischaemic events with IVUS-guided compared with angiography-guided interventions1. The clinical benefit initially emerged in more complex anatomic settings2 (i.e., long lesions or chronic total occlusions), was shown to persist up to five years following the intervention3, and was subsequently confirmed in more broadly inclusive, “all-comers” settings4. In tandem with this growing evidence base, clinical practice guidelines recommend that procedural guidance with IVUS or OCT should be considered in selected patients to optimise stent implantation5.
The value of imaging-guided PCI is increasingly appreciated, yet IVUS and OCT are still used in only a small proportion of stenting procedures in most parts of the world6. As with all diagnostic tools or therapeutic interventions, a key question arises: how can intracoronary imaging be implemented in order to maximise the anticipated clinical benefit? In this issue of EuroIntervention, Kim and colleagues report an individual patient-level pooled analysis of four contemporary RCTs that compared IVUS-guided versus angiography-guided PCI7.
The study focused on one-year clinical outcomes based on the attainment of stent optimisation criteria by IVUS. Patients (n=1,396) had undergone DES implantation for long (>26 mm) or chronically occluded lesions and were divided post hoc into two groups in relation to the achievement or non-achievement of absolute or relative stent expansion criteria (minimal stent area [MSA] ≥5.5 mm2 or ≥80% of the reference mean lumen area [MLA], respectively). The first notable finding is that, even in a controlled RCT setting, an optimal result according to the (conservative) expansion criteria was met in less than 60% of patients. Of prognostic relevance, patients in whom these expansion cut-offs were not met had a threefold higher adjusted risk of the primary endpoint (a composite of cardiac death, myocardial infarction [MI], stent thrombosis [ST], or target vessel revascularisation), along with a 2.7-fold higher risk of the “harder” composite of cardiac death, MI or ST. Both absolute and relative stent underexpansion were predictors of major adverse cardiac events (MACE); patients who fulfilled neither the relative nor the absolute stent expansion cut-offs had the worst outcomes7. Limitations of the study include the small number of events (particularly hard events), the one-year follow-up, and the complex anatomic setting such that generalisability to simpler coronary lesions remains unclear.
What does this report add to the current evidence in the field? Comprehensive analysis of available studies demonstrates that intracoronary imaging guidance reduces cardiovascular mortality and MACE compared with angiography-guided PCI8. The present study7 goes a step further by pointing to the prognostic importance of attaining quantitative optimisation standards, thereby reinforcing the concept that the use of IVUS is not an “intention-to-treat” tool. Mere performance of an IVUS pullback with visual confirmation of what may look like a “good” result does not suffice to ensure a favourable clinical prognosis; rather, procedural guidance with IVUS is mostly useful if an objectively defined “optimal” stenting result can be achieved. In a post hoc analysis of the IVUS-XPL trial (which contributed more than half of all patients to the present pooled analysis), a suboptimal IVUS-guided PCI procedure was essentially the same as angiographic guidance in terms of occurrence of MACE within one year2. Along the same lines, in the all-comers ULTIMATE trial the one-year rate of target vessel failure was lowest in patients who did have an IVUS-guided intervention with an optimal PCI result (1.6%) and was comparably high in patients who did not meet the IVUS criteria (4.4%) or those who underwent angiography-guided interventions (5.4%)4. Collectively, assessing IVUS for PCI guidance as a binary variable (yes/no) does correlate with a significant reduction in subsequent clinical events (particularly target lesion revascularisation), but the potential benefit is diluted when all patients who undergo an imaging-guided intervention are lumped together irrespective of whether an “optimal” stenting result is achieved or not (Figure 1).
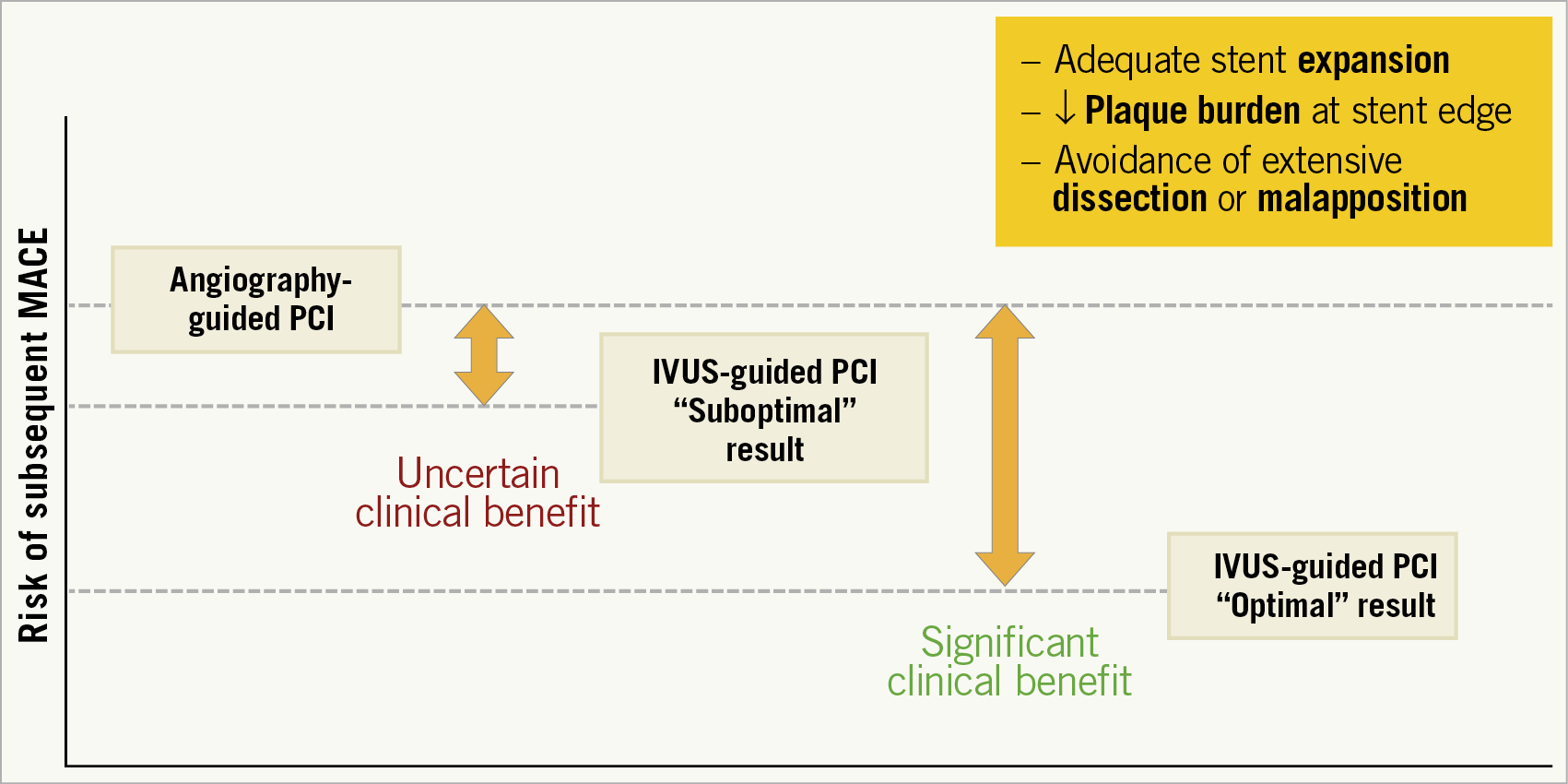
Figure 1. Post-stenting outcomes following angiography-guided or IVUS-guided interventions.
As in all post-procedural outcomes, whether an intervention results in incremental benefit is in part definition-dependent. In this respect, determining the clinical sequelae of IVUS-guided PCI in the context of specific targets is closely linked to cut-offs applied to define an “optimal” stenting result. In the analysis by Kim et al7, one needs to keep in mind that the applied criteria were not pre-specified and were in fact heterogeneous across the four included trials (with no mandated optimisation goals in two of them). Notably, the protocol-defined criteria in the largest of these four trials, IVUS-XPL (MSA greater than the luminal cross-sectional area at the distal reference)2, were not the same as the post hoc criteria used in the present pooled analysis7. The latter criteria were chosen because they represent the targets proposed in a recent expert consensus document by the EAPCI9 and were identified by Kim and colleagues as the best predictive combination criteria of absolute and relative stent expansion in their sizeable pooled data set. Thereby, this analysis provides support for the consensus-based EAPCI targets, which were acknowledged by the expert group to reflect a rather conservative approach compared with more stringent goals in several IVUS or OCT trials (e.g., MSA >90% of the average reference lumen area)9. It should also be noted that additional features of an “optimal” stenting result that may affect outcomes (including residual plaque at the stent edge, major dissection, extensive protrusion or malapposition) were not considered in the present analysis7. In a broader perspective, defining imaging targets that will optimally balance between yielding a satisfactory post-stenting result (thus reducing the risk of future MACE) versus avoiding overly aggressive post-dilatation to achieve excessively ambitious goals (which may increase the risk of prognostically relevant complications) requires further investigation. This could be addressed either by exploratory analyses of available RCT data sets or, ideally, in prospective, properly designed and adequately powered new trials.
As the totality of evidence unequivocally supports the value of intracoronary imaging for PCI guidance, it is becoming increasingly clear that the achievement of objectively defined criteria is key to ensuring better post-stenting outcomes for our patients. Correct interpretation and appropriate reaction to the imaging findings, based on standardised quantitative measurements and practical optimisation algorithms9, should be encouraged. Pragmatically, even if it may not always be possible in real-world practice to attain a combination of strict goals for an “optimal” result, it is reasonable to assume that this approach will prompt operators to pursue the best achievable result in each individual case.
Conflict of interest statement
The author has no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

