
Abstract
Aims: Our aim was to assess whether intravascular ultrasound (IVUS) improves clinical outcomes during implantation of first- and second-generation drug-eluting stents (DES). IVUS guidance is associated with improved clinical outcomes during DES implantation, but it is unknown whether this benefit is limited to either first- or second-generation devices.
Methods and results: MEDLINE, EMBASE and PubMed were searched for studies comparing outcomes between IVUS- and angiography-guided PCI. Among 909 potentially relevant studies, 15 trials met the inclusion criteria. The primary endpoint was MACE, defined as death, myocardial infarction, target vessel/lesion revascularisation (TVR/TLR) or stent thrombosis (ST). Summary estimates were obtained using Peto modelling. In total, 9,313 patients from six randomised trials and nine observational studies were included. First-generation DES were implanted in 6,156 patients (3,064 IVUS-guided and 3,092 angiography-guided) and second-generation in 3,157 patients (1,528 IVUS-guided and 1,629 angiography-guided). IVUS guidance was associated with a significant reduction in MACE (odds ratio [OR] 0.73, 95% CI: 0.64-0.85, p<0.001), across both first- (OR 0.79, 95% CI: 0.67-0.92, p=0.01) and second-generation DES (0.57, 95% CI: 0.43-0.77, p<0.001). For second-generation DES, IVUS guidance was associated with significantly lower rates of cardiac death (OR 0.33, 95% CI: 0.14-0.78, p=0.02), TVR (OR 0.47, 95% CI: 0.28-0.79, p=0.006), TLR (OR 0.61, 95% CI: 0.42-0.90, p=0.01) and ST (OR 0.31, 95% CI: 0.12-0.78, p=0.02). Cumulative meta-analysis highlighted progressive temporal benefit towards IVUS-guided PCI to reduce MACE (OR 0.60, 95% CI: 0.48-0.75, p<0.001).
Conclusions: IVUS guidance is associated with a significant reduction in MACE during implantation of both first- and second-generation DES platforms. These data support the use of IVUS guidance in contemporary revascularisation procedures using second-generation DES.
Introduction
Percutaneous coronary intervention (PCI) and stenting is an established therapeutic option for patients with stable angina and confers prognostic advantage when performed in the context of acute coronary syndromes. However, recurrent major adverse cardiovascular events (MACE) following successful stenting remains an ongoing clinical problem. Although the introduction and widespread uptake of the drug-eluting stent (DES) contributed to significant reductions in the need for repeat revascularisation, ~20% of patients will re-present with further symptoms within five years following PCI1. Thus, strategies that can improve clinical outcomes are of great importance.
Intravascular ultrasound (IVUS) use during PCI is known to impact on interventional strategy by providing important information on target lesion and reference vessel characteristics. Following stent deployment, IVUS can accurately quantify stent expansion and strut apposition, and may identify stent edge-related complications not always apparent on angiography2. Although previous meta-analyses have shown that IVUS-guided DES implantation is associated with a significant reduction in MACE, the results were either predominantly based on studies in first-generation DES3,4 or were limited to randomised trials5. However, the majority of PCI procedures performed worldwide use second-generation DES, often in “off-label” indications, where the role of IVUS remains uncertain.
We sought to assess whether the clinical benefit of IVUS-guided DES implantation is maintained in second-generation devices by performing a systematic review and meta-analysis, stratified by DES generation.
Methods
DATA SOURCES AND SEARCH STRATEGY
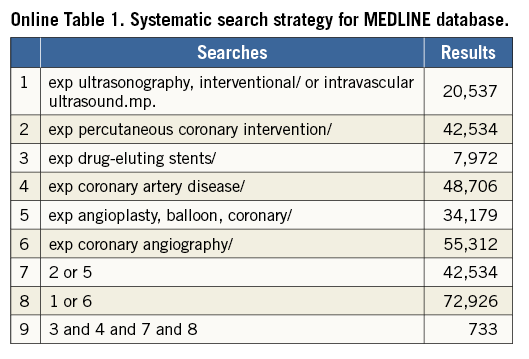
A keyword search was performed through the MEDLINE, EMBASE and PubMed databases for the period January 2000 to May 2016. Keywords using Medical Subject Heading included: “intravascular ultrasound”, “ultrasound, intravascular”, “percutaneous coronary intervention”, “drug-eluting stents”, “coronary artery disease”, “coronary angioplasty” and “coronary angiography” with no limits set (Online Table 1). The reference lists of selected articles were also reviewed for relevant citations. Abstract lists of major international conferences were also manually searched to identify other potential sources of data. Conferences included were the European Society of Cardiology, EuroPCR, American College of Cardiology Scientific Sessions, American Heart Association Scientific Sessions and Transcatheter Cardiovascular Therapeutics. The study was registered with the PROSPERO international register (CRD42016037632) and adhered to the PRISMA statement6.
STUDY SELECTION
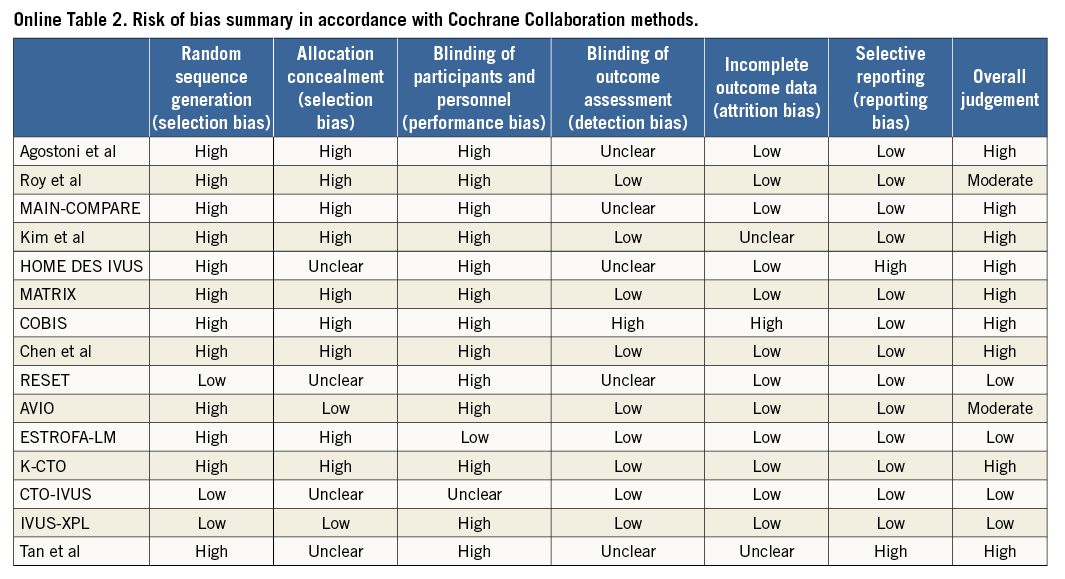
Two investigators (A.J. Brown and N. Nerlekar) independently conducted the literature search, and two further investigators (C.J. Cheshire and K.P. Verma) performed data extraction for study demographics, design, angiographic characteristics and clinical outcomes. Randomised clinical trials, observational and registry studies were included. Other specific inclusion criteria required reported clinical outcomes at a minimum of six months, comparison of IVUS- and angiography-guided strategies and differentially reported outcomes for first- and second-generation stents. For observational studies, matched propensity data were included in the final analysis, where available. First (or early)-generation stents were defined as either paclitaxel (e.g., TAXUS™; Boston Scientific, Marlborough, MA, USA) or sirolimus (e.g., CYPHER®; Cordis, Johnson & Johnson, Miami Lakes, FL, USA) eluting, durable polymer, metallic stents with thick strut design, while second (or new)-generation stents were defined as either zotarolimus (Endeavor®; Medtronic, Minneapolis, MN, USA) or everolimus (XIENCE V®; Abbott Vascular, Santa Clara, CA, USA, or PROMUS™; Boston Scientific, Marlborough, MA, USA) eluting, durable polymer, metallic platforms with a strut thickness <100 µm. A.J. Brown and N. Nerlekar verified extracted data and discrepancies for included studies. Where studies included both stent generations, corresponding authors were contacted to provide individual outcomes if available. The remaining studies with mixed stent platforms were excluded. The risk of bias within individual studies was assessed according to the Cochrane Collaboration Assessment (Online Table 2).
CLINICAL ENDPOINTS
The primary endpoint of this study was MACE, with secondary endpoints including cardiac death, non-fatal myocardial infarction (MI), target lesion revascularisation (TLR), target vessel revascularisation (TVR), and stent thrombosis. The definition of MACE differed slightly across trials, but generally included death, MI and TVR. Two studies included cardiac and non-cardiac death7,8, while seven reported cardiac death9-15 and four all-cause death16-19. Six studies used TLR instead of TVR8,13,14,17-19, while stent thrombosis was included as part of MACE in two studies12,13. MACE could not be calculated from the reported data of two studies20,21, but these studies contributed to individual clinical endpoints. Trial-specific definitions of MACE were used in the analysis. MI was defined as either Q-wave or non-Q-wave MI. Stent thrombosis was defined as definite or probable according to the Academic Research Consortium22.
STATISTICAL ANALYSIS
Statistical analysis was performed by using STATA MP 13.0 (StataCorp LP, College Station, TX, USA). Outcomes were analysed using a Peto model as a small number of events were expected, especially in randomised controlled trials. Sensitivity analysis was also performed using a DerSimonian and Laird random effects model and inverse variance method with a fixed model which did not differ from the Peto model. Summary statistics are reported as a pooled odds ratio (OR) with 95% confidence intervals (CI) between first- and second-generation DES for the outcome of MACE. Further sensitivity analysis was also performed comparing only RCTs between first- and second-generation stents. Additional subgroup analysis was performed to evaluate summary statistics for various components of MACE (all-cause death, cardiac death, MI, TVR, TLR and stent thrombosis). Finally, we performed a cumulative meta-analysis to evaluate the impact of publication date on the summary odds ratios and to assess temporal trends in effect size. Statistical heterogeneity was quantified with the I2 statistic. Heterogeneity was quantified as low, moderate or high based on I2 values of 25%, 50% and 75%, respectively23. Publication bias was assessed by the Harbord test between first- and second-generation studies24. A two-sided p-value of <0.05 was considered significant.
Results
A total of 909 publications were reviewed, with 32 studies selected for potential inclusion and further evaluation. Of these studies, 17 were excluded as they included bare metal stents or their reported outcomes included both first- and second-generation DES25-42. The authors of two studies provided individualised outcome data stratified by DES generation and these were included8,11. This resulted in 15 studies meeting the predefined inclusion criteria (Figure 1).
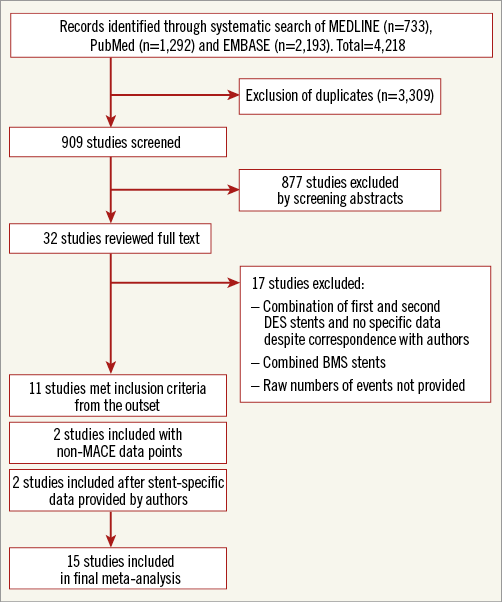
Figure 1. Flow diagram of study. Flow diagram illustrating the identification and screening of eligible studies. BMS: bare metal stent; DES: drug-eluting stent; MACE: major adverse cardiovascular events
Six studies in the analysis were randomised controlled trials, including the HOME DES IVUS study17, the AVIO trial10, the RESET trial12, the IVUS-XPL trial14, CTO-IVUS15 and one randomised trial of unprotected left main coronary artery stenting in the elderly19. The other nine were observational studies assessing the use of IVUS in specific lesion subsets, including left main coronary artery stenting7,8,20, bifurcation PCI11,18,21 or chronic total occlusions13, while two were consecutive patient registries9,16. Baseline characteristics of the included studies are presented in Table 1.
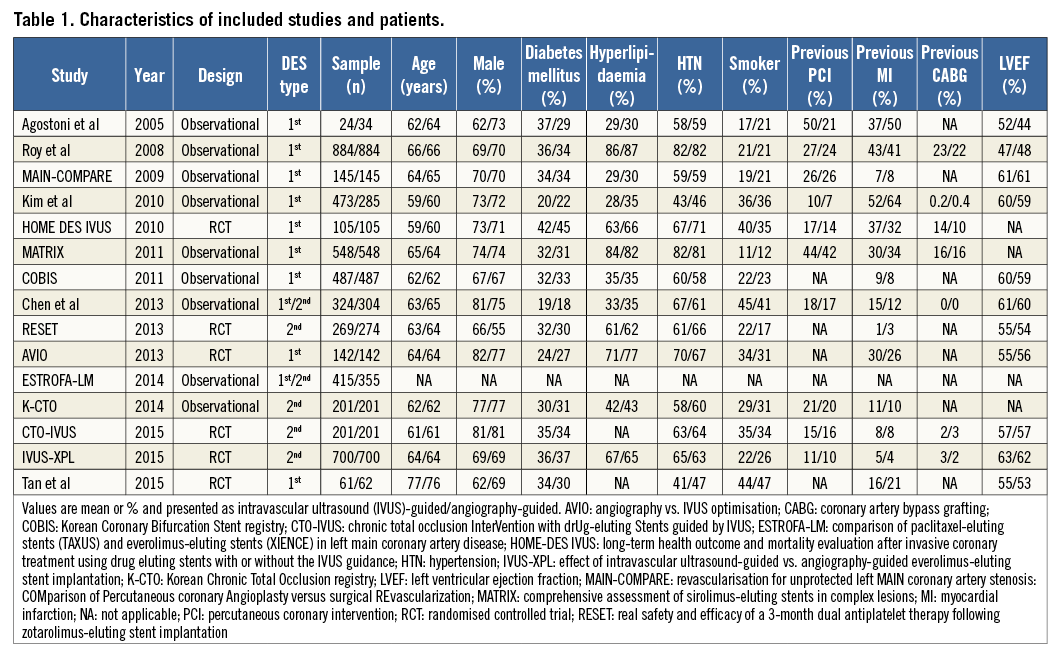
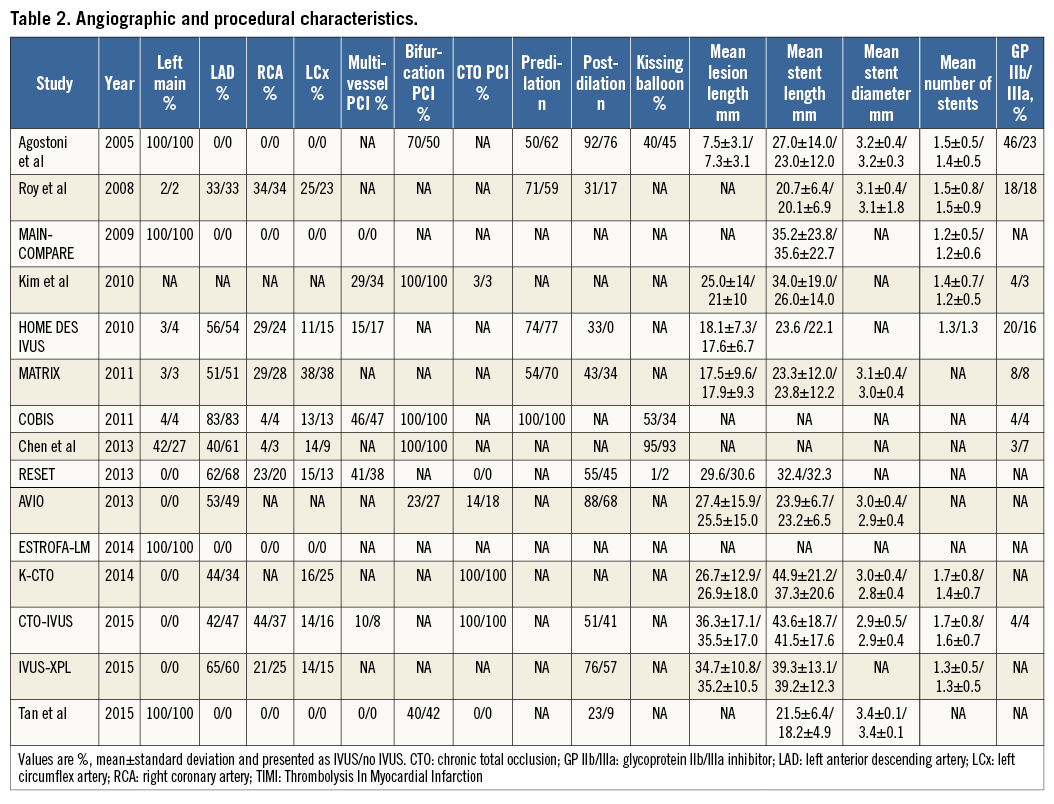
Of the 9,313 patients included, 6,156 received a first-generation DES and 3,157 received a second-generation DES. For first-generation DES, 3,064 patients underwent IVUS-guided PCI and 3,092 angiography-guided PCI, while for second-generation DES 1,528 patients underwent IVUS-guided PCI and 1,629 angiography-guided PCI. Angiographic and procedural characteristics of the included studies are presented in Table 2. Data on IVUS-guided PCI for first-generation DES were available for eleven studies7-11,16-21, while data on IVUS-guided PCI for second-generation DES were available from six studies8,11-15.
MAJOR ADVERSE CARDIOVASCULAR EVENTS
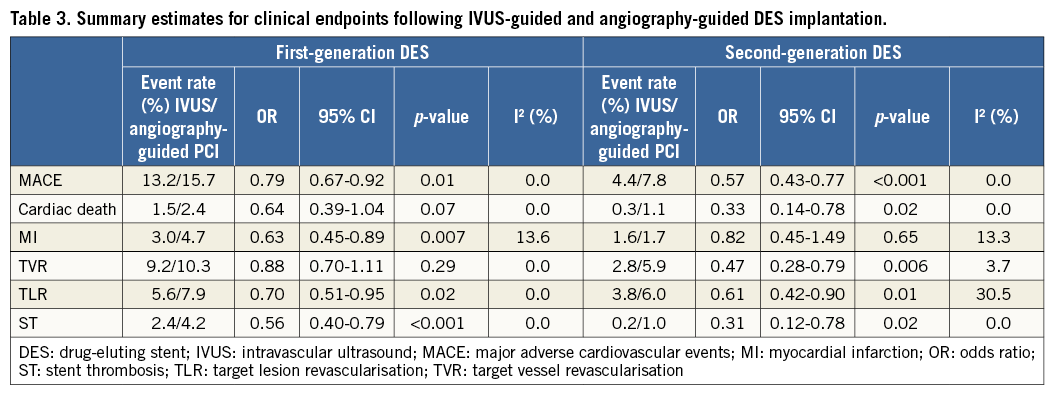
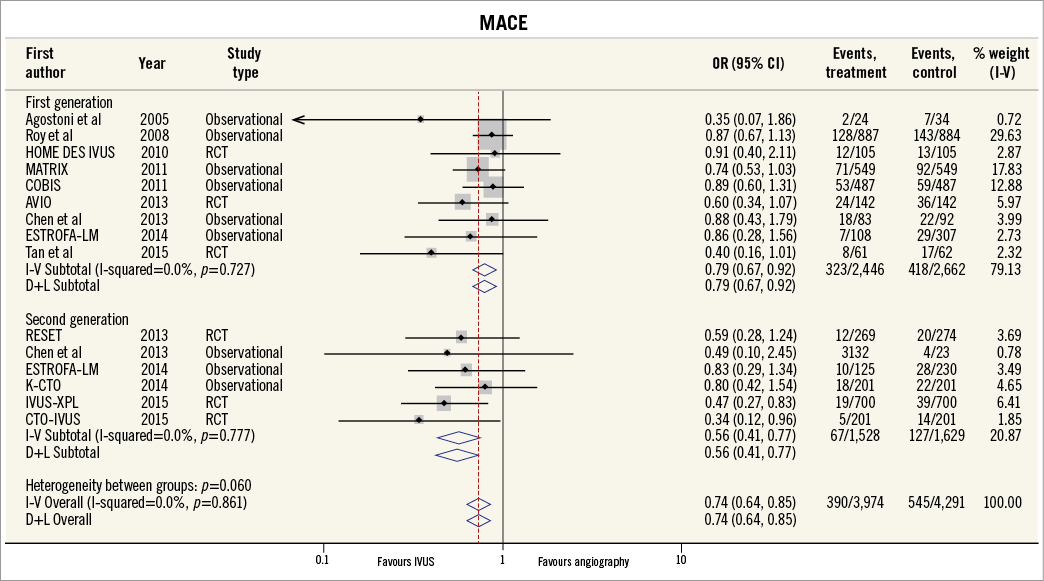
Thirteen of the included studies reported the incidence of MACE. The summary OR for all studies was 0.73 (95% CI: 0.64-0.85, p<0.001) in favour of IVUS-guided PCI (Figure 2). There was no evidence of statistical heterogeneity between studies (I2=0%). The beneficial effect of IVUS-guided PCI was observed for both first-generation (OR 0.79, 95% CI: 0.67-0.92, p=0.01, I2=0%) and second-generation DES (OR 0.57, 95% CI: 0.43-0.77, p<0.001, I2=0%). No difference was noted with inverse variance and random-effects modelling (Online Figure 1), and there was no significant interaction between first- and second-generation stent groups (p=0.06). The potential for publication bias was assessed statistically by the Harbord test, which demonstrated no evidence of small study effects, either in first-generation (p=0.08) or in second-generation DES studies (p=0.73). Sensitivity analysis limited to randomised trials was consistent with these findings, with IVUS-guided PCI associated with reduced MACE in both first-generation (OR 0.62, 95% CI: 0.41-0.93, p=0.02, I2=0.0%) and second-generation DES (OR 0.49, 95% CI: 0.34-0.72, p<0.001, I2=0.0%) (Online Figure 2, Online Figure 3).
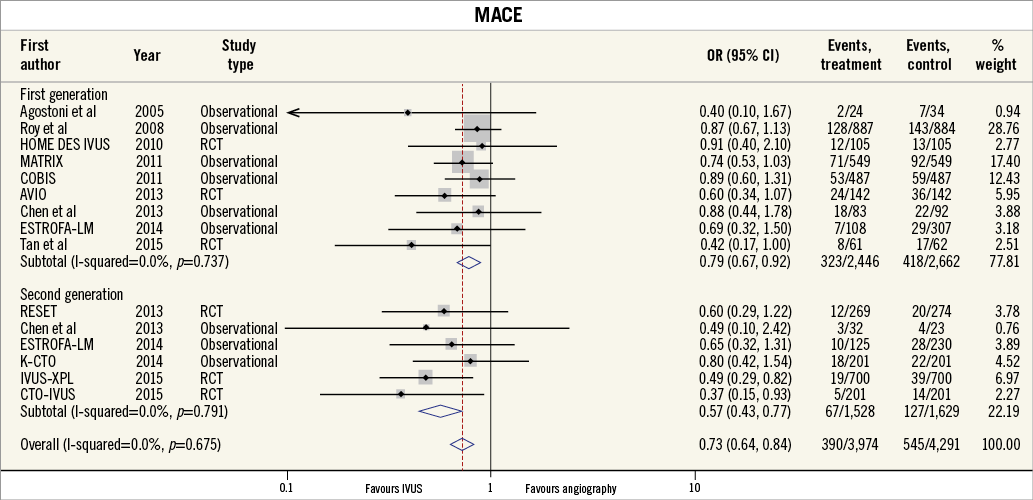
Figure 2. MACE for IVUS-guided versus angiography-guided PCI stratified by DES generation. The incidence and odds ratios (OR) for major adverse cardiovascular events (MACE) following both intravascular ultrasound (IVUS)-guided and angiography-guided drug-eluting stent (DES) implantation. The benefit of IVUS-guided PCI is consistent across both first- and second-generation DES. CI: confidence interval; RCT: randomised controlled trial
CARDIAC DEATH AND MYOCARDIAL INFARCTION
The incidence of cardiac death was reported in nine studies, while ten studies reported the incidence of MI. IVUS-guided PCI was associated with a significant reduction in the risk of cardiac death (OR 0.55, 95% CI: 0.36-0.83, p=0.005, I2=0%). This benefit appeared limited to second-generation DES (OR 0.33, 95% CI: 0.14-0.78, p=0.02, I2=0%), with no clear benefit in first-generation DES (OR 0.64, 95% CI: 0.39-1.04, p=0.07, I2=0%) (Figure 3). IVUS-guided PCI was also associated with a significant reduction in the risk of MI (OR 0.67, 95% CI: 0.50-0.90, p=0.01, I2=8.6%). IVUS-guided PCI did not appear to reduce the risk of MI in second-generation DES (OR 0.82, 95% CI: 0.45-1.49, p=0.65, I2=13.3%), but did for first-generation DES (OR 0.63, 95% CI: 0.45-0.89, p=0.007, I2=13.6%).
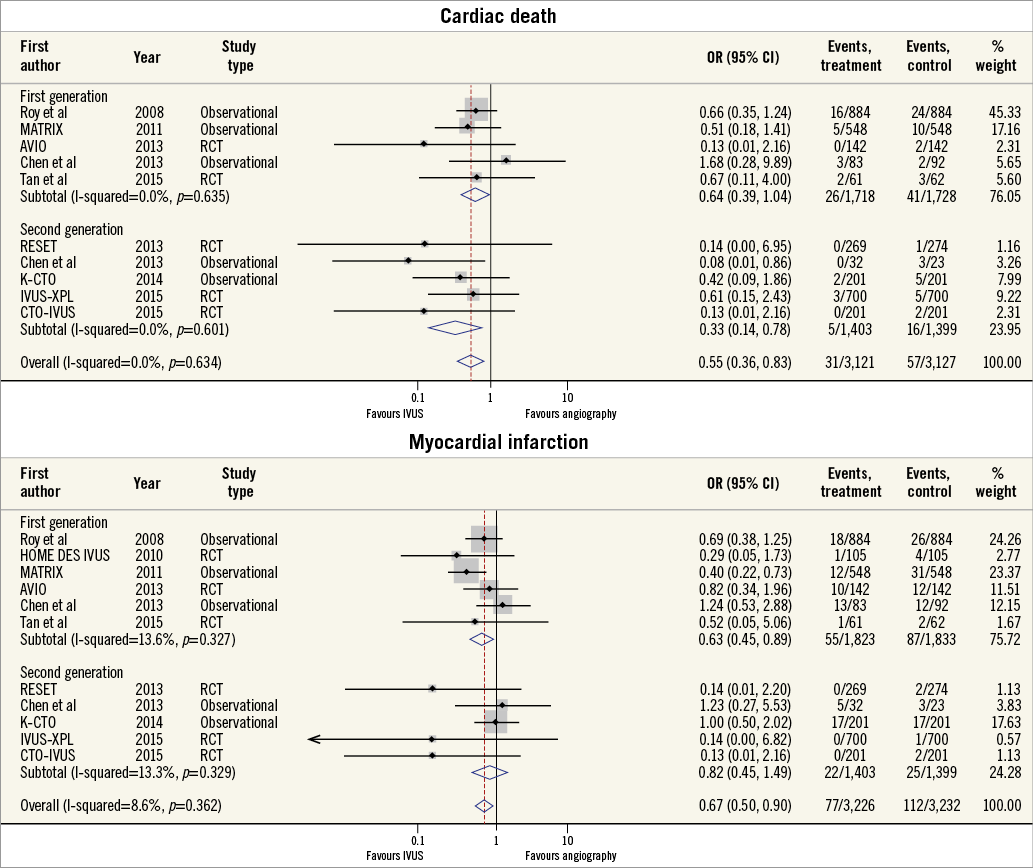
Figure 3. Summary plot for clinical endpoints of cardiovascular mortality and myocardial infarction. The incidence and odds ratios (OR) for cardiac death (A) and myocardial infarction (B) following both intravascular ultrasound (IVUS)-guided and angiography-guided drug-eluting stent (DES) implantation, stratified by drug-eluting stent generation. CI: confidence interval; RCT: randomised controlled trial
OTHER CLINICAL ENDPOINTS
Seven studies reported the incidence of TVR and eight studies reported the incidence of TLR. Overall, IVUS-guided PCI significantly reduced the risk of TVR (OR 0.79, 95% CI: 0.64-0.98, p=0.04, I2=23.2%) and TLR (OR 0.66, 95% CI: 0.52-0.84, p<0.001, I2=0.0%) (Figure 4). Although IVUS-guided PCI reduced the incidence of both TVR and TLR in second-generation DES, only TLR was reduced in first-generation DES (Table 3). IVUS-guided PCI significantly reduced the risk of ST across both first- (OR 0.56, 95% CI: 0.40-0.79, p<0.001, I2=0.0%) and second-generation DES (OR 0.31, 95% CI: 0.12-0.78, p=0.02, I2=0.0%).
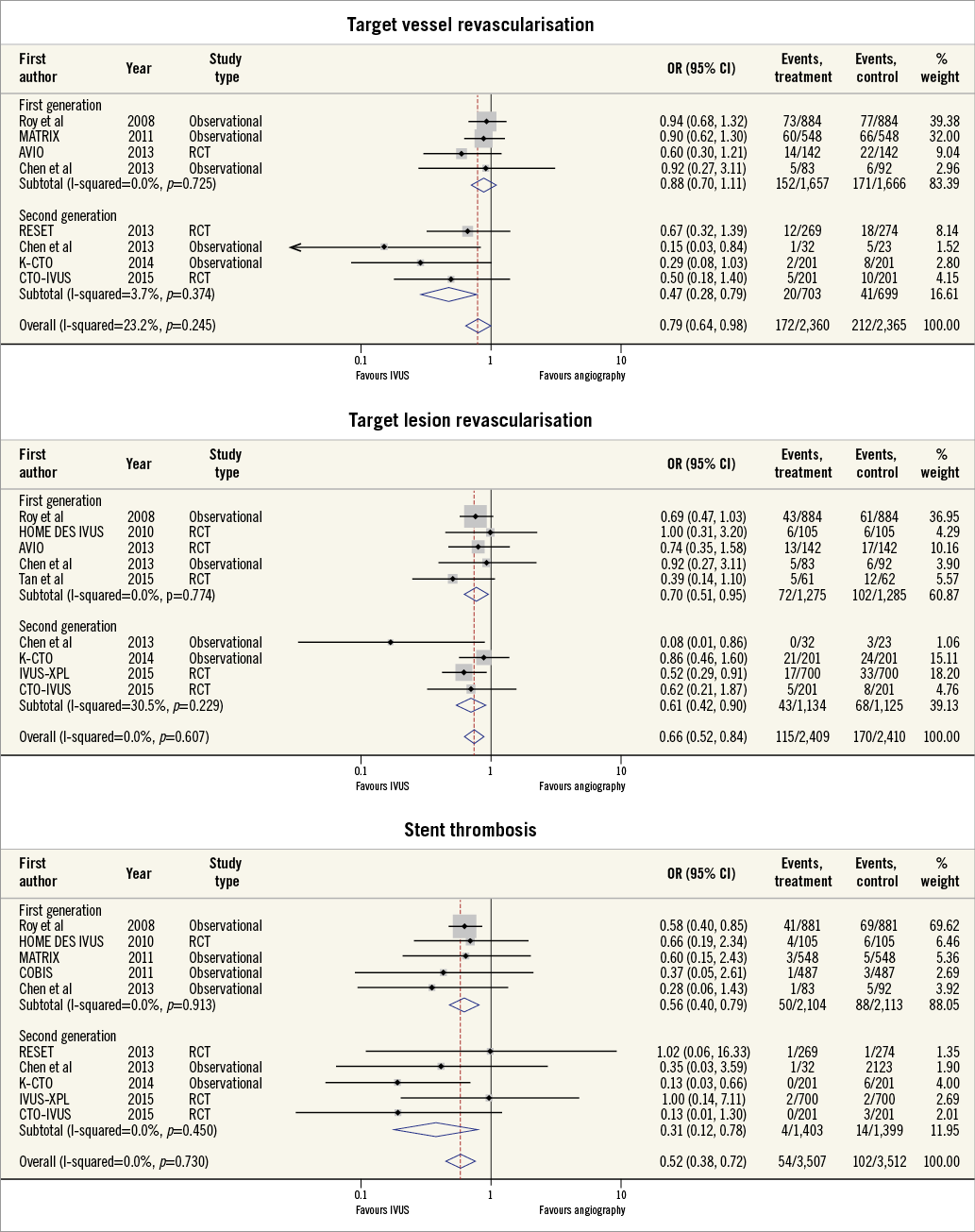
Figure 4. Summary plot for stent-specific endpoints of target vessel/lesion revascularisation and stent thrombosis. The incidence and odds ratios (OR) for target vessel revascularisation (A), target lesion revascularisation (B) and stent thrombosis (C) following both intravascular ultrasound (IVUS)-guided and angiography-guided drug-eluting stent (DES) implantation, stratified by drug-eluting stent generation. CI: confidence interval; RCT: randomised controlled trial
CUMULATIVE META-ANALYSIS
Finally, we performed a cumulative meta-analysis on the whole cohort, assessing for temporal trends in the effectiveness of IVUS-guided PCI (Figure 5). Until the end of 2011 (total of 4,111 patients), the summary OR was 0.83 (95% CI: 0.69-0.99, p=0.04). However, after this date there was an incremental progressive trend towards a greater beneficial effect of IVUS-guided PCI to reduce MACE (total of 4,154 patients), with a summary OR of 0.60 (95% CI: 0.48-0.75, p<0.001).
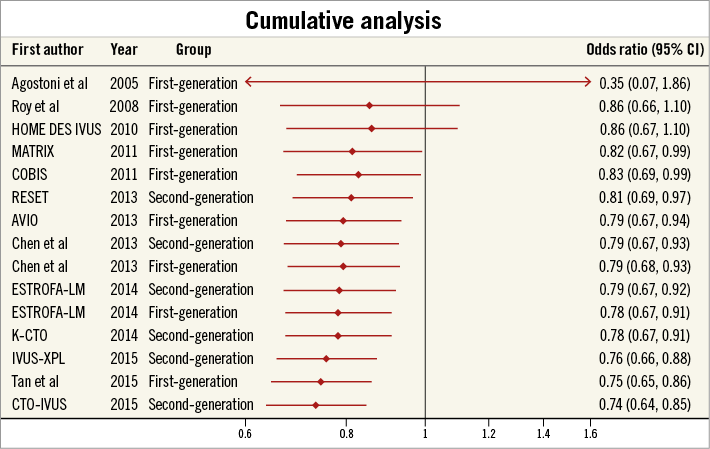
Figure 5. Cumulative meta-analysis by year of study publication. Cumulative meta-analysis with odds ratios for major adverse cardiovascular events (MACE) by year of study publication showing a continual and progressive benefit of IVUS-guided DES implantation.
Discussion
Our results demonstrate that IVUS-guided DES implantation is associated with a significant reduction in MACE, when compared with angiography-guided PCI. Importantly, the benefit of an IVUS-guided PCI strategy applies across both first- and second-generation DES. In second-generation DES, IVUS guidance reduces the incidence of cardiac death and clinical endpoints related to DES failure. Finally, we show a temporal and progressive improvement in clinical outcomes for IVUS-guided DES implantation. These results should continue to encourage the use of IVUS-guided PCI and reinforce that clinical benefits are maintained independent of DES type.
Previous meta-analyses have reported that IVUS-guided DES implantation is associated with significantly lower rates of MACE, with an OR of between 0.60 and 0.753-5. However, these three reports included studies using a mixture of DES types and there remains uncertainty on the clinical utility of an IVUS-guided strategy in newer platforms. Here we show that an IVUS-guided second-generation DES implantation is associated with an ~40% relative risk reduction of MACE, when compared with an angiography-guided strategy. Importantly, the majority of the benefit we observed for IVUS guidance with second-generation DES was in clinical endpoints of stent failure, including TVR, TLR and ST (relative risk reductions of 55%, 46% and 80%, respectively). Based on these data, we find that the number needed to treat using IVUS to prevent one MACE event during second-generation DES implantation is ~30 (95% CI: 19.7-57.0). Cost-effectiveness analyses are now required to assess whether the current threshold for IVUS use is appropriate or whether operators should be expanding use to more “routine” cases.
Our results also suggest that there has been a continual temporal improvement in the reported clinical outcomes for studies assessing the role of IVUS guidance during DES implantation. Although definitive mechanisms underlying this observation cannot be provided by the current analysis, this evolutionary effect may be a consequence of operators becoming increasingly aware of what constitutes optimal stent deployment. The use of IVUS allows operators to evaluate stent expansion quantitatively, with underexpansion independently associated with repeat revascularisation and ST43. Additionally, the continual improvement in IVUS catheter technology and image resolution now makes it easier for operators to identify small edge-related complications of PCI, including inflow/outflow dissections and geographical miss of plaque, which have potential to impact on long-term prognosis2,44. Further trials that refine our definitions of IVUS-guided optimal stent deployment are now required to ensure treatment benefits are consistent across interventional institutions.
Limitations
There are some limitations to this study that should be highlighted. Firstly, the definitions of MACE differed among studies. However, the clinical benefit of IVUS-guided PCI was observed across all study endpoints and we observed no statistical heterogeneity for the outcome of MACE among studies. Secondly, we were unable to obtain trial-specific DES outcomes for 17 studies identified by our systematic search criteria, and inclusion of these data may have affected the final results. Third, most studies in the current analysis were observational studies. In an effort to ensure that our results were robust, we used propensity-matched data when available, with a sensitivity analysis limited to randomised controlled trial data showing similar findings.
Conclusions
IVUS guidance is associated with a significant reduction in MACE when utilised during PCI with both first- and second-generation DES platforms. These data should support the use of IVUS-guided PCI for contemporary revascularisation procedures, especially in patient and lesion subsets that are known to have a worse clinical prognosis.
| Impact on daily practice IVUS-guided PCI is known to improve clinical outcomes during implantation of DES, particularly in the treatment of complex lesion subsets. However, there remains uncertainty as to whether this beneficial effect is limited to first-generation DES platforms. These data suggest that IVUS guidance is associated with a significant reduction in adverse events during implantation of both first- and second-generation DES, supporting IVUS use during the contemporary procedures performed in daily clinical practice. |
Acknowledgements
The authors would like to thank Dr Jose M. de la Torre Hernandez, Dr Nai-Liang Tian and Prof. Shao-Lian Chen for providing unpublished study data included in this manuscript.
Funding
L. McCormick is supported by a Robertson Family Research Cardiologist Fellowship.
Conflict of interest statement
I. Meredith has acted on the Scientific Advisory Board of Boston Scientific. The other authors have no conflicts of interest to declare.
Supplementary data
Online Figure 1. MACE for IVUS-guided versus angiography-guided PCI stratified by DES generation using random-effects model and inverse variance method.
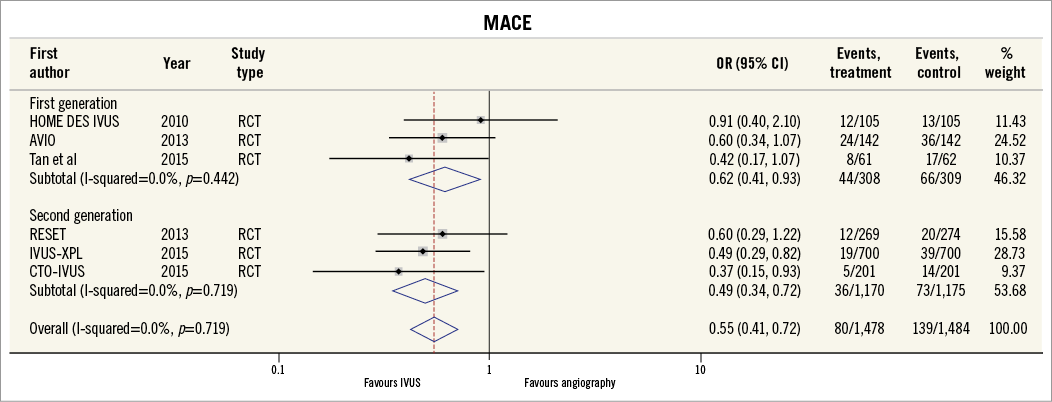
Online Figure 2. MACE for IVUS-guided versus angiography-guided PCI in randomised controlled trials stratified by DES generation using Peto model.
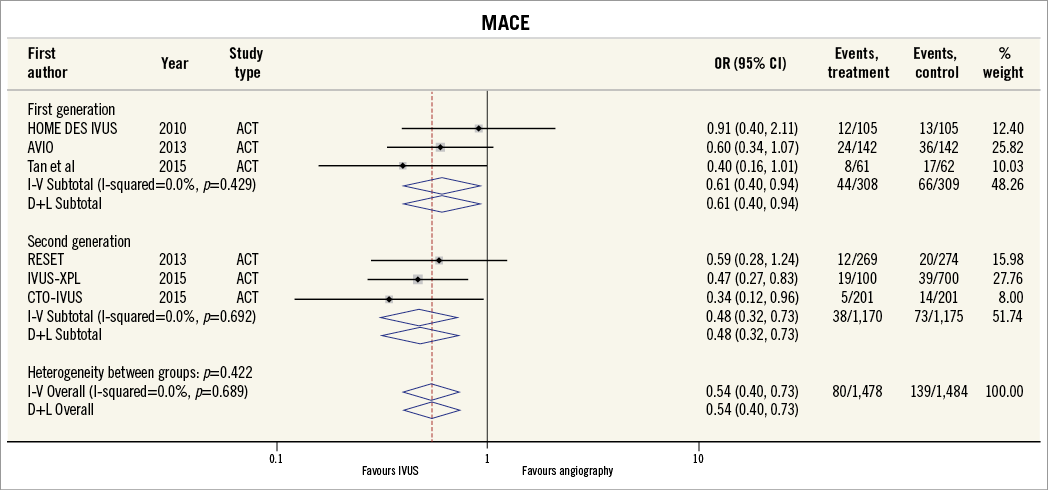
Online Figure 3. MACE for IVUS-guided versus angiography-guided PCI in randomised controlled trials stratified by DES generation using random-effects model and inverse variance method.

