Abstract
Aims: The impact of intravascular ultrasound (IVUS) guided coronary drug-eluting stent (DES) implantation on clinical outcomes remains controversial. A meta-analysis of the currently available clinical trials investigating IVUS-guided DES implantation was undertaken.
Methods and results: We searched Medline, the Cochrane Library and other internet sources, without language or date restrictions, for published articles comparing clinical outcomes between IVUS-guided and angiography-guided DES implantation. Clinical studies with both adjusted and unadjusted data were included. Eleven studies were identified (one randomised controlled trial and 10 registries) and included in the meta-analysis with a weighted follow-up time of 20.7±11.5 months. Compared with angiography guidance, IVUS-guided DES implantation was associated with a reduced incidence of death (hazard ratio [HR]: 0.59, 95% confidence interval [CI]: 0.48-0.73, p<0.001), major adverse cardiac events (HR: 0.87, 95% CI: 0.78-0.96, p=0.008) and stent thrombosis (HR: 0.58, 95% CI: 0.44-0.77, p<0.001). The incidence of myocardial infarction (HR: 0.82, 95% CI: 0.63-1.06, p=0.126), target lesion (HR: 0.90, 95% CI: 0.73-1.11, p=0.316) and target vessel (HR: 0.90, 95% CI: 0.77-1.05, p=0.195) revascularisation was comparable between the angiography and IVUS-guided arms. A repeat meta-analysis of propensity-matched studies only (six studies, n=5,300) yielded broadly similar results in terms of clinical outcomes.
Conclusions: IVUS-guided coronary DES implantation is associated with a significant reduction in death, MACE and stent thrombosis compared to angiography guidance. Appropriately powered randomised trials are necessary to confirm the findings from this meta-analysis.
Introduction
Intravascular ultrasound (IVUS) is a catheter-based invasive imaging technique that provides high resolution cross-sectional images of the lumen and vessel wall and allows for the reliable quantification of the luminal, outer vessel wall dimensions and plaque burden1. The unique advantages of IVUS have rendered it an indispensable tool in the assessment of new invasive intracoronary treatments. In the coronary angioplasty era, IVUS has proven to be an invaluable tool in advancing the understanding of mechanisms of restenosis (vessel wall negative remodelling and intima proliferation)2,3, findings which subsequently aided in the development of intracoronary stents. In the early era of intracoronary bare metal stents (BMS), several IVUS-based studies were conducted to identify predictors of subacute stent thrombosis and optimal stenting techniques4-6. IVUS-guided BMS implantation was shown to improve acute procedural results and reduce major adverse cardiac events (MACE), predominantly through a reduction in restenosis and need for repeat revascularisation7,8.
In the drug-eluting stent (DES) era, IVUS aided in optimising implantation factors for intracoronary DES, and helped to identify mechanisms of in-stent restenosis and late stent thrombosis (LST)9,10. Multiple studies have shown that stent underexpansion, residual reference segment stenosis, incomplete stent apposition and coronary dissection after DES implantation are potentially associated with an increased risk of stent thrombosis and MACE11,12. In spite of the accumulating evidence in the last decade suggesting that IVUS-guided stent implantation can optimise DES deployment, no sufficiently powered randomised controlled trial has been undertaken to confirm the clinical value of IVUS-guided DES implantation. In this meta-analysis we systematically analyse the published clinical studies investigating IVUS-guided DES implantation to investigate the potential clinical value of such an approach.
Methods
DATA SOURCES AND SEARCH STRATEGY
We conducted a computerised search of Medline, the Cochrane Library and internet sources for clinical studies from January 1995 to August 2012 using the medical subject heading terms “ultrasound, intravascular”, “angiography”, “drug-eluting stent”, as well as a combination of the terms “IVUS-guided”, “angiography-guided”, and “drug-eluting stents”. We used the Science Citation Index as a cross-reference to identify studies that met the search criteria. PubMed was searched using the method described by Biondi-Zoccai et al13. Additional searches for potential studies included the references of review articles, and earlier meta-analyses.
STUDY SELECTION AND DATA EXTRACTION
The studies selected for inclusion in the meta-analysis met the following predetermined criteria: (1) they were clinical studies published with full data available in peer-reviewed journals; (2) the studies included a comparison of the efficacy and safety of IVUS-guided and angiography-guided DES implantation; and (3) the follow-up time was at least six months. Studies that simultaneously investigated BMS and DES without separate clinical outcomes for the DES subgroup were excluded from this meta-analysis, with the exception of one study23. Within this study23 (n=8,173) patients predominantly underwent DES implantation, with BMS implanted in only a small proportion of the study population (IVUS-guided arm: 2.4%, angiography-guided arm: 7.4%). It was therefore deemed appropriate to include this study in the meta-analysis. Filtering by all of the above criteria yielded 11 clinical studies.
ENDPOINTS
The endpoints in the analysis included: (1) all-cause death (in one study22 that recruited 58 patients all-cause death was not reported and cardiovascular mortality data was included instead), (2) major adverse cardiac events (MACE), (3) stent thrombosis (ST), (4) myocardial infarction (MI) (non-Q-wave MI and Q-wave MI), (5) target lesion revascularisation (TLR), and (6) target vessel revascularisation (TVR). Three independent reviewers (MHL, SHY and HGG) extracted the data, which included the study’s first author name, the sample size, the patients’ baseline characteristics, the follow-up duration, and the clinical outcomes in the angiography-guided and IVUS-guided groups.
PROPENSITY SCORE MATCHING GROUP SUB-ANALYSIS
Similar analyses were undertaken using studies that included propensity score matching sub-analyses18-21,26,27.
STATISTICAL ANALYSES
The baseline characteristics were analysed with weighted means for continuous variables using two-sample Student t-tests, and weighted proportions for categorical variables using chi-square tests, to compare the IVUS-guided and angiography-guided groups. Hazard ratios (HR) and 95% confidence intervals (CI) as relevant effects were estimated by the given data directly or indirectly. Summary statistical data were extracted from the included studies according to the standard methods for survival endpoints, with HR and CI as preferred sources for estimation14. Standard techniques for meta-analysis were used to calculate the pooled estimates15. A negative HR indicated an advantage for IVUS-guided interventions, whereas a positive HR indicated a benefit for angiography-guided interventions. A random effect model of the meta-analysis was used to aggregate outcomes and calculate the 95% CI. The heterogeneity of the studies was assessed using chi-square tests (p>0.1 indicated that there was no significant heterogeneity between the studies) and I2 tests (I2 >25%, >50%, and >75% indicated that there was low, moderate and severe heterogeneity respectively, particularly in small sample studies). All p-values were two-tailed with the statistical significance set at <0.05. Forest plots were generated for graphical presentations of the clinical outcomes. Funnel plots were conducted by plotting the precision against the log HR or against the mean difference to assess the presence of publication or reporting bias. In addition Egger’s linear regression analysis was computed as previously described16. All analyses were conducted using STATA 12.0 (Stata Corp., College Station, TX, USA).
Results
STUDIES INCLUDED
Eleven studies were included in this meta-analysis17-27. Ten studies18-27 were observational registries and one study17 was a randomised trial (Figure 1). Six studies had propensity-based matching of baseline characteristics18-21,26,27. The study characteristics of each trial are shown in Table 1.
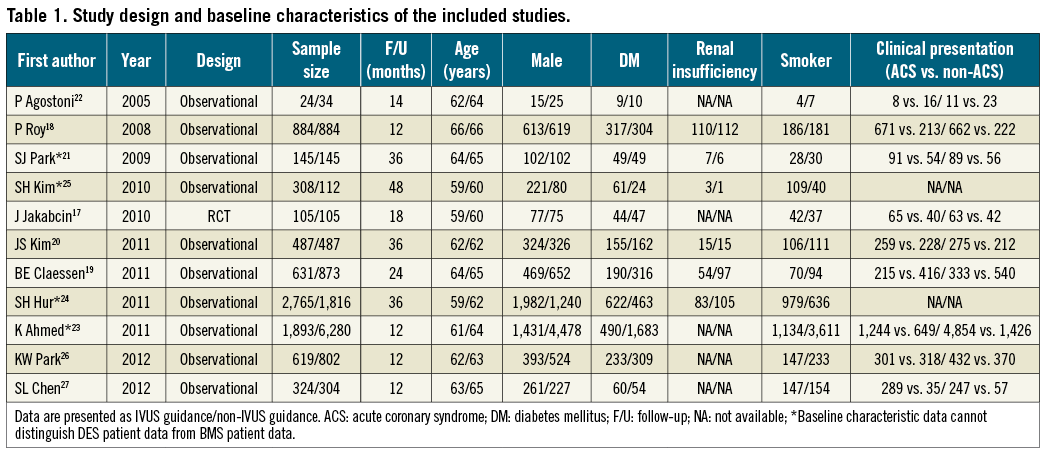
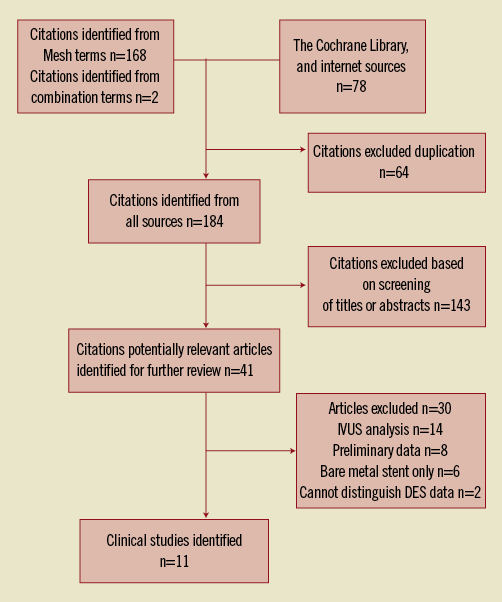
Figure 1. Flow diagram of the process followed to identify the relevant studies that were entered in the current meta-analysis.
PATIENT CHARACTERISTICS
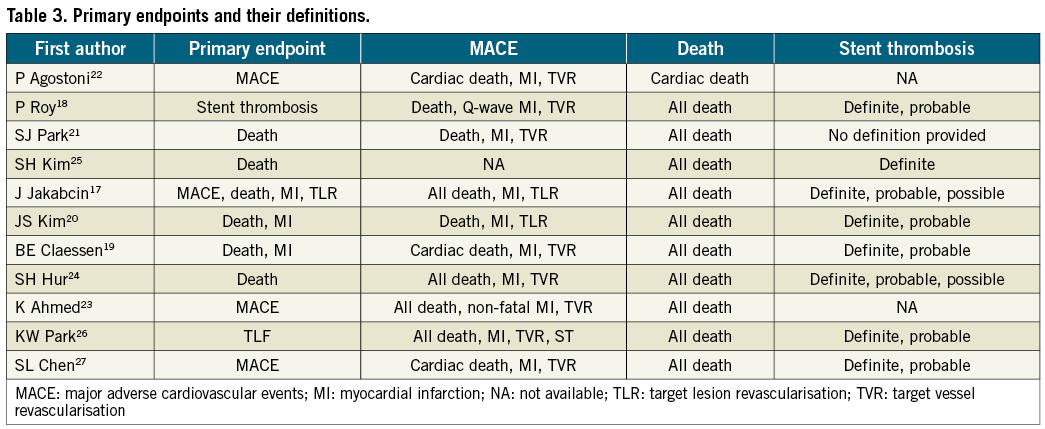
Data was analysed from 8,102 patients (41.3%) who underwent IVUS-guided DES implantation and 11,517 patients (58.7%) who underwent angiography-guided DES implantation (overall patient numbers, n=19,619). Comparisons of baseline characteristics between the IVUS-guided and the angiography-guided groups demonstrated no statistical significant differences in gender (72% vs. 71%, p=0.28) and the prevalence of diabetes mellitus (25% vs. 27%, p=0.12) (Table 1). Patients with angiography-guided PCI were older (63.6 years vs. 61.3 years, p<0.05), had more frequent renal insufficiency (7% vs. 5%, p<0.05), involved more intervention because of acute coronary syndrome (ACS) (62% vs. 71%, p<0.05) and were more likely to smoke (36% vs. 44%, p<0.05). Patients’ follow-up ranged from six to 48 months, with a weighted follow-up time of 20.7±11.5 months. Table 2 demonstrates the angiographic characteristics of the treated lesions in the two groups. Table 3 shows the primary endpoints of the studies that were included in the current meta-analysis and the definitions used to describe cardiovascular events.
CLINICAL OUTCOMES
The HR and 95% CI for the studies’ outcomes are presented in Figure 2. Standard deviations are calculated from the investigated studies of the clinical outcomes: consequently, the 95% CI will marginally differ from that reported in the original data.
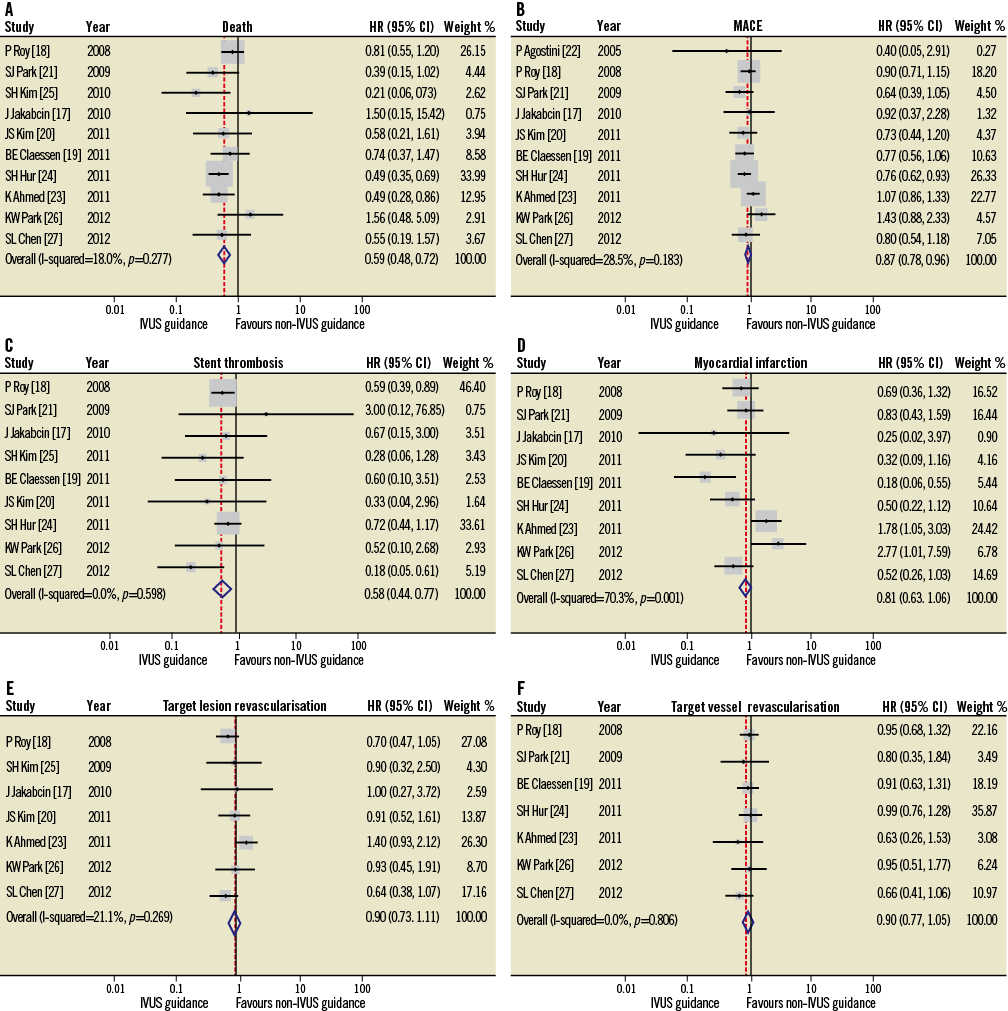
Figure 2. HR and conclusion plots for death (A), MACE (B), stent thrombosis (C), myocardial infarction (D), target vessel (E) and target lesion (F) revascularisation associated with IVUS vs. non-IVUS guidance DES implantation.
DEATH
Ten studies reported all-cause mortality and one study reported cardiovascular death. IVUS-guided DES implantation was associated with a significantly lower mortality rate (HR 0.59, 95% CI: 0.48-0.73, p<0.001; Figure 2A). The chi-square test (p=0.28) and I2 test (17.9%) did not indicate significant heterogeneity within the studies.
MAJOR ADVERSE CARDIOVASCULAR EVENTS (MACE)
MACE was reported in 10 of the 11 studies that were included in the current meta-analysis. IVUS-guided DES implantation was associated with a significant reduction in MACE (HR 0.87, 95% CI: 0.78-0.96, p=0.008; Figure 2B). Statistical analyses indicated no significant heterogeneity (p=0.18, I2=28.5%).
STENT THROMBOSIS (ST)
Nine studies reported the incidence of ST at follow-up and indicated that IVUS-guided DES implantation led to a significant reduction in the incidence of ST (HR 0.58, 95% CI: 0.44-0.77, p<0.001; Figure 2C). No evidence of statistical heterogeneity was noted between these studies (chi-square test, p=0.60; I2 statistics, I2=0%).
MYOCARDIAL INFARCTION (MI), TARGET LESION REVASCULARISATION (TLR) AND TARGET VESSEL REVASCULARISATION (TVR)
Seven studies reported MI, five TLR, and five TVR. The risk of MI, TLR, and TVR did not significantly differ between the IVUS-guided and angiography-guided groups (MI: HR 0.82, 95% CI: 0.63-1.06, p=0.126; TLR: HR 0.90, 95% CI: 0.73-1.11, p=0.316; TVR: HR 0.90, 95% CI: 0.77-1.05, p=0.195; Figure 2D, Figure 2E and Figure 2F). In addition, significant data heterogeneity was evident among the studies with MI as an endpoint (p=0.001, I2=70%).
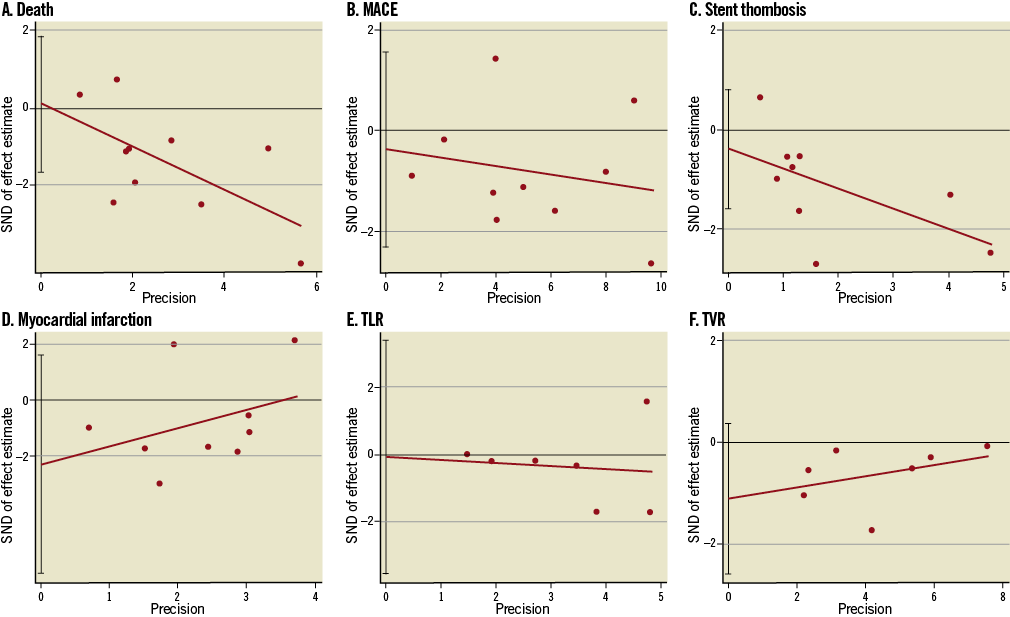
Figure 3. Funnel plots for death (A), major adverse cardiovascular events (MACE) (B), stent thrombosis (C), myocardial infarction (MI) (D), TLR (E) and TVR (F) by Egger’s linear regression analysis.
PUBLICATION BIAS
Since this meta-analysis included 11 clinical studies, we assessed the underlying publication bias using quantitative tools. Visual inspection of the funnel plots for death, MACE, ST, MI, TLR and TVR did not reveal asymmetry (data not shown). Egger’s linear regression tests based on the identified studies demonstrated no significant publication bias (p=0.90 for death, p=0.65 for MACE, p=0.46 for ST, p=0.21 for MI, p=0.96 for TLR and p=0.11 for TVR; Figure 3).
PROPENSITY SCORE MATCHING GROUP SUB-ANALYSIS
Six studies18-21,26,27 included propensity score matching sub-analysis with 5,300 patients involved (Figure 4). The clinical outcomes of death, ST and MI demonstrated a significant reduction in the IVUS-guided group compared to the angiography-guided group (HR 0.73, 95% CI: 0.54-0.99, p=0.04; HR 0.57, 95% CI: 0.39-0.84, p=0.004; HR 0.63, 95% CI: 0.43-0.91, p=0.01; respectively), with a trend towards significance for MACE (HR 0.86, 95% CI: 0.73-1.00, p=0.06). No significant differences were observed for the outcomes of TLR and TVR (HR 0.86, 95% CI: 0.73-1.00, p=0.06; HR 0.85, 95% CI: 0.63-1.14, p=0.28; HR 0.94, 95% CI: 0.75-1.17, p=0.57; respectively). In addition, there was data heterogeneity among the studies reporting the MI (p=0.02, I2=64%) and MACE (p=0.08, I2=49%) endpoints. Egger’s linear regression tests based on the propensity matching studies demonstrated that only TLR had a significant publication bias (p=0.01, Figure 5).
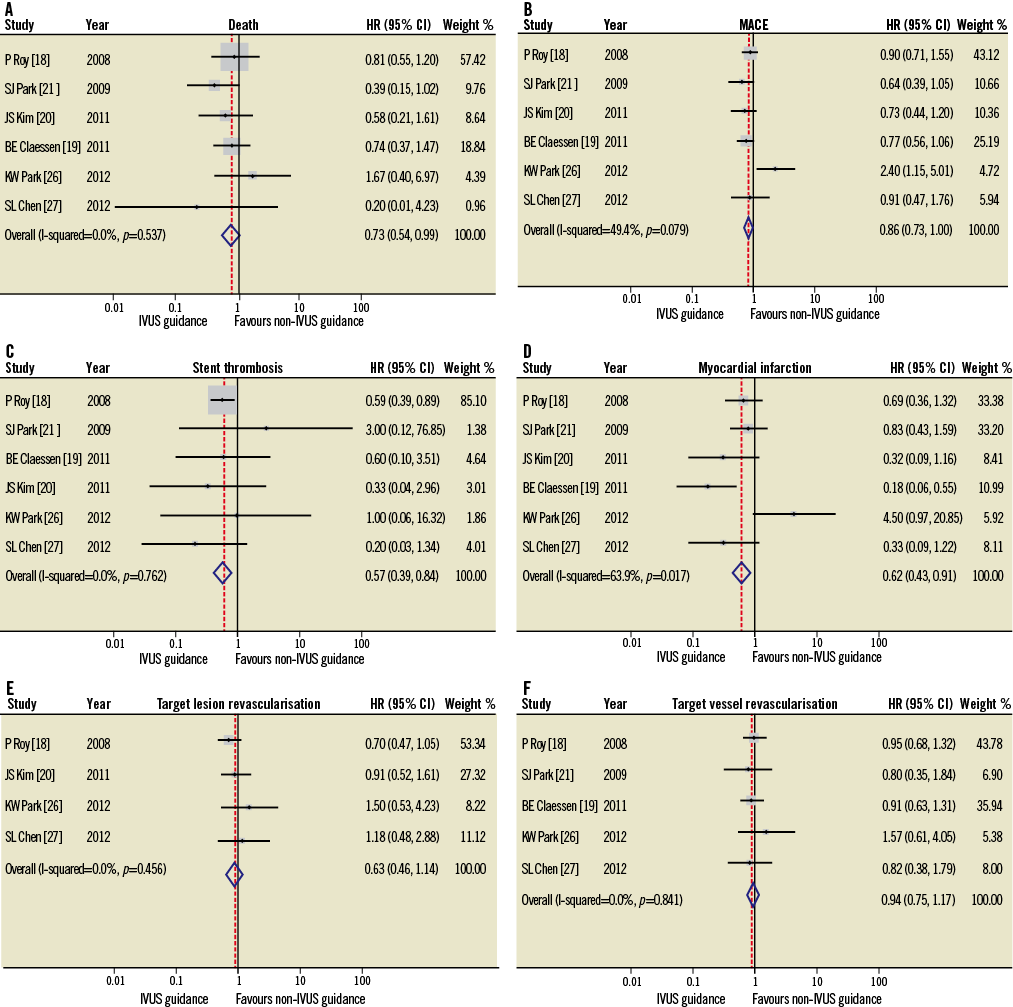
Figure 4. HR and conclusions plot for death, MACE, stent thrombosis, myocardial infarction, TLR and TVR associated with IVUS vs. non-IVUS guidance DES implantation (propensity score studies18-21,26,27, n=5,300).
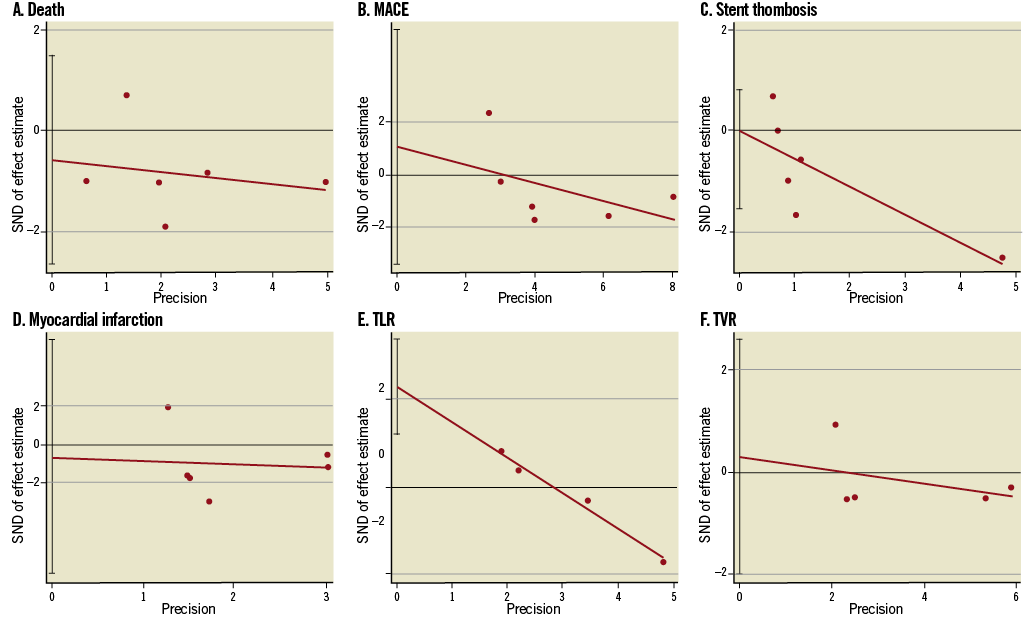
Figure 5. Funnel plots for death, MACE, stent thrombosis, myocardial infarction, TLR and TVR by Egger’s linear regression analysis (propensity score studies18-21,26,27, n=5,300).
Discussion
The present meta-analysis, which included 11 clinical studies and 19,619 patients, demonstrated that IVUS-guided DES implantation was associated with a significant reduction in mortality, MACE and stent thrombosis, findings that were broadly corroborated when similar analyses were undertaken in the meta-analysis of propensity-matched studies (six clinical studies, n=5,300).
To date, the impact of IVUS-guided DES implantation on long-term clinical outcomes has produced conflicting results17-28. This is predominantly secondary to the limited number of patients, the lack of appropriately powered randomised controlled trials17,29, a short follow-up period, heterogeneity in lesions types, and the differing presentation of patients (e.g., ACS vs. non-ACS patients). For example, the only published randomised trial investigating IVUS-guided DES implantation (Jakabcin et al17) was limited by patient numbers (n=210) and failed to demonstrate any prognostic benefit for IVUS-guided DES implantation (compared to angiographic guidance). In addition, the unpublished Angiographic versus IVUS Optimization (AVIO) randomised, multicentre trial29, investigating IVUS versus angiographic-guided DES implantation in complex lesions (142 patients in each study arm), again failed to demonstrate any differences in clinical outcomes (combined endpoint of MI, TLR, TVR, or cardiac death) at 30 days and 9 months. Despite being limited in power, the AVIO Trial reported a higher minimal lumen diameter in the IVUS-guided DES implantation group compared to the angiography-guided group (2.70 mm versus 2.51 mm, p=0.0002).
The present meta-analysis addresses some of the aforementioned limitations and provides more robust data about the potential prognostic value of IVUS-guided DES implantation. The large number of patients included (n=19,619) and the relatively long follow-up period (almost two years) potentially offer sufficient data and clinical events which allow for a detailed assessment of the value of IVUS-guided DES implantation. We found that IVUS-guided DES deployment reduced overall mortality and MACE, findings that were broadly corroborated in the propensity-matched meta-analyses (Figure 4). The improved clinical outcomes are probably related to the lower risk of ST in the IVUS-guided group reported in nine studies17-21,24-27. Notably, the definition of ST as an outcome was inconsistently recorded in eight studies17-20,24-27 and not provided in one study21 (Table 3), which may make interpretation of the outcomes related to ST more difficult. In order to overcome this limitation, analyses were repeated in six studies (n=6,307)18-20,25-27 where the definition of ST was consistent (definite/probable ST30) (data not shown). Repeat analyses using criteria for ST indicated that a significant reduction in ST remained evident in the IVUS-guided group, p<0.001). Mechanisms to explain the reduction in ST with IVUS guidance may be the superior resolution of IVUS (100 microns) compared to coronary angiography (150-200 microns) and its consequent superior ability to identify stent edge dissections and haematomas, stent underexpansion and incomplete stent apposition (all risk factors for ST), not detected by conventional coronary angiography31-33.
Concerning the timing of the beneficial effects of IVUS-guided DES implantation, this is unclear from the present meta-analysis. Only three studies reported clinical outcomes at 30 days (n=3,492)18,19,27 and they failed to show any improvement in clinical outcomes (namely MACE, death, MI, ST, TVR, TLR) (data not shown). Since the clinical events were low, the analysis was underpowered to draw any definitive conclusions for this time point.
Conversely, the present meta-analysis failed to show any differences in TVR and TLR between the IVUS and angiography-guided groups. This discrepancy may be attributed to the fact that TLR and TVR were reported in only seven of the 11 studies, as well as to the relatively short follow-up period of TLR (weighted follow-up time is 14.9±8.6 months). Furthermore, several studies had confirmed the so-called “late catch-up phenomenon”, in which neointimal proliferation is prolonged beyond the first six months after DES implantation, particularly with first-generation DES, with a subsequent higher incidence of TLR and TVR during longer-term follow-up34-37.
Recently, Prati et al38 reported the one-year follow-up clinical outcomes of the CLI-OPCI study (Angiography alone versus angiography plus optical coherence tomography [OCT] to guide decision-making during PCI). Patients undergoing PCI with OCT guidance at three high-OCT-volume Italian centres between 2009 and 2011 were investigated (n=670). The OCT guidance group (n=335 consecutive patients) was matched 1:1 with the angiography guidance group (n=335 randomly selected subjects undergoing PCI during the same month with only angiographic guidance). Although baseline and angiographic characteristics in the two groups remained unbalanced after matching, following the extensive multivariable analyses adjusting for baseline and procedural differences, the OCT-guided group was shown to be associated with a lower risk of cardiac death or MI (OR:0.59, 95% CI: 0.25-0.96, p=0.037). The findings of this preliminary study assessing OCT-guided PCI lend further support to the use of intravascular imaging to guide PCI and the need for larger, prospectively run, sufficiently powered randomised controlled trials.
Limitations
Although the present meta-analysis provides evidence that the use of IVUS during DES implantation improves clinical outcomes, there are significant limitations which should be taken into consideration during the interpretation of the reported findings. Firstly, the present meta-analysis was based on registries that examined the use of IVUS in different settings and included unadjusted patient data. Hence, the presence of data heterogeneity is likely to have affected the final results. For example, the meta-analysis included patients with left main coronary artery disease. It is not inconceivable that the reported benefit may have been driven in part by the left main revascularisation, given that one propensity-based study suggested a possible mortality benefit21. Given the lack of patient-level data in the present meta-analysis, it was not possible to exclude all the patients who had left main PCI, as most registries included such patients. Secondly, lesion pathology dictates a different therapeutic approach, e.g., more post-dilatation in the case of long lesions or bifurcation lesions vs. direct stenting without post-dilatation in acutely occluded coronary vessels. Furthermore, in patients presenting with acute MI, IVUS has been shown not to improve clinical outcomes23: this may be attributed to the fact that IVUS guidance often requires more post-dilatation with the subsequent risk of distal embolisation39. Thirdly, there were differences in the baseline characteristics between the IVUS-guided and the angiography-guided groups, namely that more patients in the angiography-guided group had ACS and were smokers. Lastly, different DES (namely early and new-generation DES) may also impact on the incidence of ST: since most of the included data could not distinguish between DES types, such an analysis could not be performed40.
Although it is not possible to overcome these limitations, as almost all the imported registries were heterogeneous, we have attempted to reduce this effect by the reporting of a separate meta-analysis including only propensity-matched studies (Figure 4). In order to account for the possibility of left main disease or ACS potentially confounding the data, the two studies21,22 that recruited only patients with left main stem disease (n=348) and the one study23 that included only patients treated with ACS (n=8,173) were excluded in a repeated meta-analysis (n=11,098)17-20,24-27 (Online appendix). Within this separate meta-analysis, the IVUS-guided and the angiography-guided groups had similar baseline characteristics, apart from older patients and more renal insufficiency in the angiography-guided arm. In this sub-analysis the results obtained were not substantially different from those reported in the main analysis, with the additional finding of a significantly lower incidence of MI and TLR in the IVUS-guided PCI group.
All of the present study findings therefore confirm the potential value of IVUS-guided DES implantation, specifically in the treatment of more complex lesion types41,42, and highlight the need to conduct large-scale sufficiently powered randomised trials with adequate follow-up periods.
Conclusions
The current meta-analysis investigates the prognostic value of IVUS-guided DES implantation. IVUS-guided DES implantation is associated with a significant reduction in death, MACE and ST. These results underline the potential value of IVUS in the treatment of coronary artery disease, particularly in complex procedures. Appropriately powered randomised trials are necessary to confirm the reported findings and to identify the types of lesions and the populations that would benefit most from IVUS-guided DES implantation.
Conflict of interest statement
The authors have no conflicts of interest to declare.
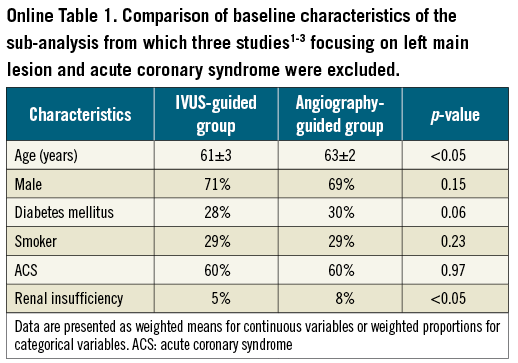
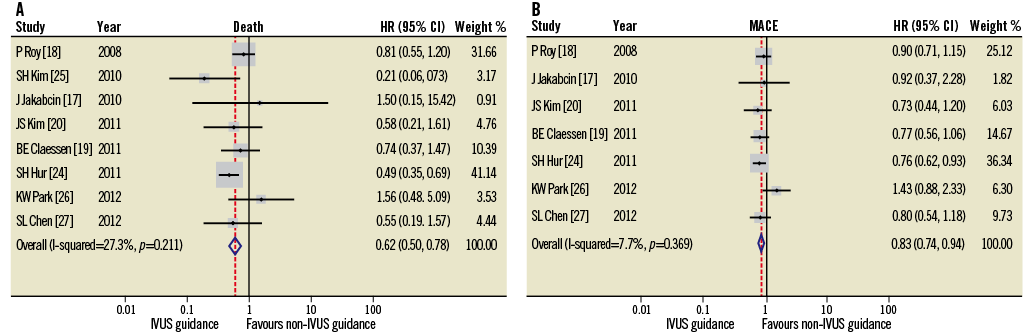
Online Figure 1. Hazard ratios (HR) and conclusions plots for death (A) and MACE (B) associated with IVUS vs. non-IVUS guidance DES implantation (selected studies4-11, n=11,098).
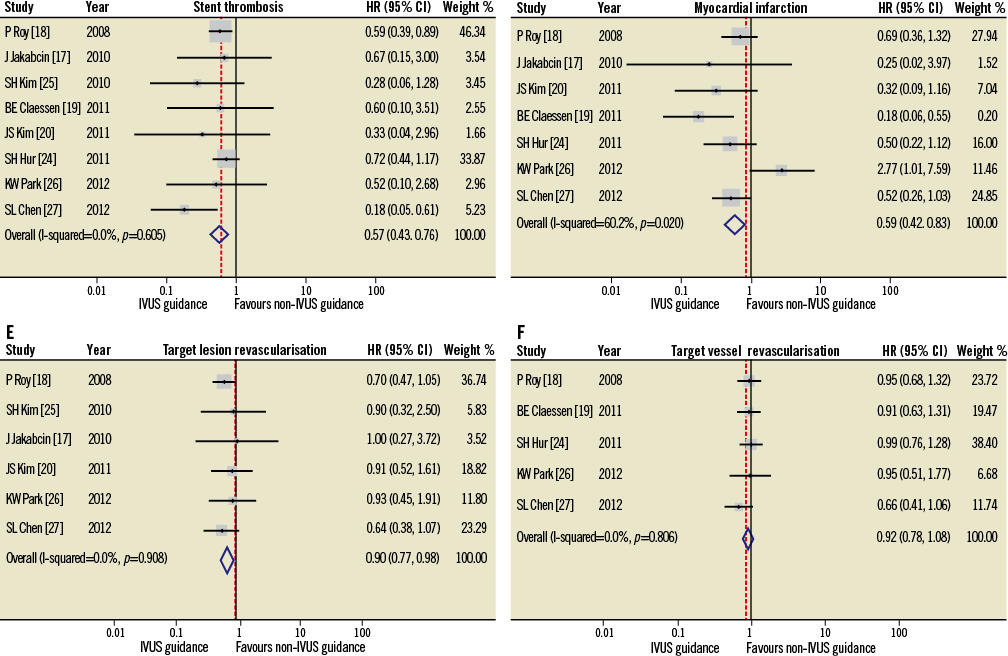
Online Figure 2. Hazard ratios (HR) and conclusions plots for stent thrombosis (A), myocardial infarction (B), target vessel (C) and target lesion (D) revascularisation associated with IVUS vs. non-IVUS guidance DES implantation (selected studies4-11, n=11,098).
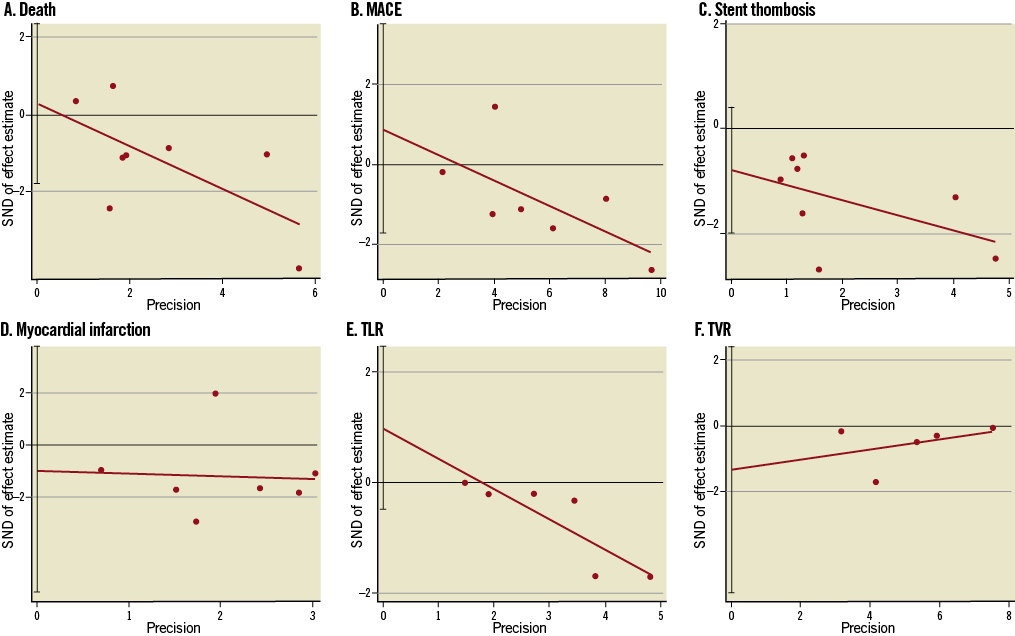
Online Figure 3. Funnel plots for death (A), major adverse cardiovascular events (MACE) (B), stent thrombosis (C), myocardial infarction (MI) (D), TLR (E) and TVR (F) by Egger’s linear regression analysis (selected studies4-11, n=11,098). MACE: major adverse cardiovascular events; TLR: target lesion revascularisation; TVR: target vessel revascularisation
Online references

