Recently, we have published the manuscript entitled “Comparison of intravascular ultrasound versus angiography-guided drug-eluting stent (DES) implantation: a meta-analysis of one randomised trial and ten observational studies involving 19,619 patients”. Two additional important studies (namely, the ADAPT-DES and the RESET), comparing IVUS- and angiography-guided DES implantation have recently been reported1,2. In addition, the two-year clinical follow-up of the randomised AVIO trial was published recently3. Therefore, our meta-analysis needs to be updated.
The ADAPT-DES (Assessment of Dual AntiPlatelet Therapy with Drug-Eluting Stents) trial was a prospective multicentre registry study that enrolled approximately 11,000 patients1. The outcomes, after IVUS-guided percutaneous coronary intervention (PCI), were compared to the non-IVUS-guided PCI group in 8,575 patients. A significant reduction in the primary endpoint of definite/probable stent thrombosis was evident in patients who underwent PCI under IVUS guidance (vs. angiography guidance) at one-year follow-up (0.52% vs. 1.04%, p=0.011). In the pre-specified long lesion subset of the RESET (Real Safety and Efficacy of a 3-month Dual Antiplatelet Therapy Following Zotarolimus-eluting Stents Implantation) trial2, 543 patients were enrolled and randomised to either the IVUS- or angiography-guided PCI group. IVUS-guided PCI was related to a significantly lower risk of major adverse cardiac events (MACE) at one-year follow-up compared to angiographic guidance (4.0% vs. 8.1%, p=0.048). In the AVIO trial, no statistical differences were observed in MACE (16.9% vs. 23.2%) or target lesion revascularisation (TLR, 9.2% vs. 11.9%) between the IVUS- and angiography-guided groups3.
Based on the available data, the currently updated meta-analysis includes 14 studies involving 29,029 patients (Figure 1)1-4. The revisited meta-analysis not only confirms our previous findings that IVUS guidance was associated with reductions in death (hazard ratio [HR]: 0.66, 95% confidence interval [CI]: 0.55-0.78, p<0.001), stent thrombosis (HR: 0.57, 95% CI: 0.44-0.73, p<0.001), myocardial infarction (MI) (HR: 0.74, 95% CI: 0.62-0.90, p=0.002) and MACE (HR: 0.86, 95% CI: 0.77-0.95, p=0.003), but also shows the beneficial effect of IVUS guidance in reducing TLR (HR: 0.82, 95% CI: 0.68-0.97, p=0.02). Since the present meta-analysis included mostly observational studies, no significant publication bias was identified using Egger’s linear regression test (p=0.50 for death; p=0.85 for ST; p=0.69 for MI, p=0.33 for MACE, p=0.67 for TLR). Although the present meta-analysis supports and strengthens the previously reported results, appropriately powered randomised trials are necessary to provide robust evidence and verify the practical value of IVUS-guided DES implantation.
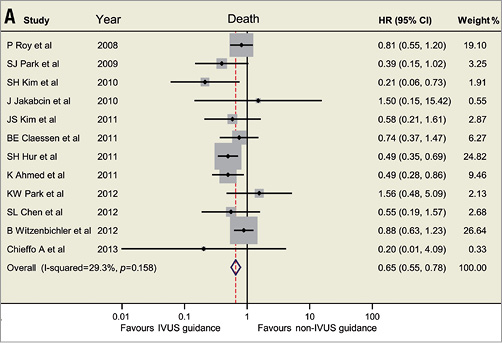
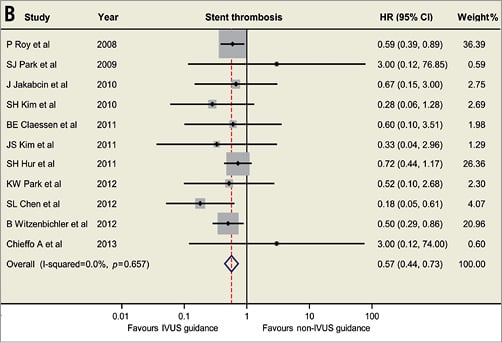
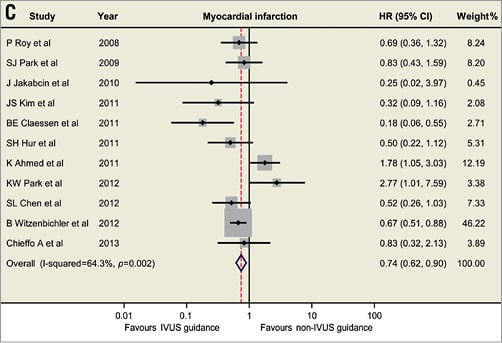
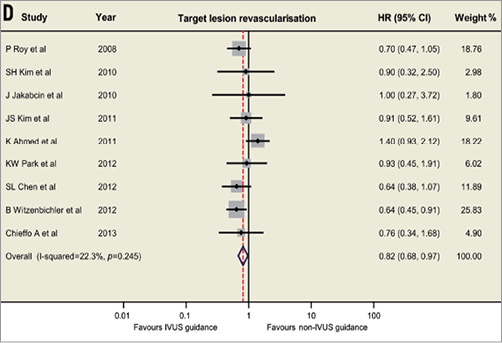
Figure 1. Hazard ratios and forest plots for (A) death, (B) stent thrombosis, (C) myocardial infarction, and (D) target lesion revascularisation associated with IVUS- versus non-IVUS-guided drug-eluting stent implantation. CI: confidence interval; HR: hazard ratio; IVUS: intravascular ultrasound
Conflict of interest statement
The authors have no conflicts of interest to declare.

