CASE SUMMARY
BACKGROUND: A 67-year-old, hypertensive, male smoker presented to our institution complaining of right-sided intermittent claudication. Background history was notable for insulin-treated diabetes mellitus and multivessel coronary artery disease with previous acute coronary syndrome. Six months earlier, the patient had undergone percutaneous transluminal angioplasty with stenting for symptomatic, long-segment proximal to mid right superficial femoral artery stenoses.
INVESTIGATION: Laboratory test, Doppler ultrasonography, angiography, computed tomography.
DIAGNOSIS: Superficial femoral artery in-stent restenosis.
TREATMENT: Paclitaxel-eluting balloon angioplasty.
KEYWORDS: Paclitaxel-eluting balloon, peripheral intervention, restenosis, stent
PRESENTATION OF THE CASE
A 67-year-old, hypertensive, male smoker presented to our institution complaining of right-sided intermittent claudication. Background history was notable for insulin-treated diabetes mellitus, with acceptable glycaemic control (glycated haemoglobin 5.7%), as well as diffuse coronary artery disease with previous acute coronary syndrome. Of note, he underwent several percutaneous coronary revascularisations due to recalcitrant drug-eluting stent (DES) in-stent restenoses.
Six months earlier, the patient had undergone percutaneous transluminal angioplasty (PTA) for symptomatic, long-segment proximal to mid right superficial femoral artery (SFA) stenoses. At that time, due to extensive arterial dissection after angioplasty, the patient was treated with self-expandable nitinol stent implantation (7.0×120 mm; 7.0×120 mm; 7.0×80 mm, SMART stent; Cordis, Miami Lakes, FL, USA) to enable an optimal acute outcome.
On admission, the patient complained of symptomatic rapidly worsening right claudication (Rutherford Class 3, walking distance <200 metres). Ultrasonography revealed right SFA occlusion at the inflow with potential for percutaneous revascularisation. For this reason, angiography was scheduled. Arterial access was via an antegrade right common femoral puncture using a 6 Fr 10 cm sheath (Terumo, Somerset, NJ, USA). Diagnostic angiography confirmed restenotic stent occlusion in the SFA at the level of the ostium (lesion Type C according to TransAtlantic Inter-Society Consensus II scheme)1 with reperfusion at the level of the distal SFA by means of collateral flow (Figure 1A and Figure 1B).
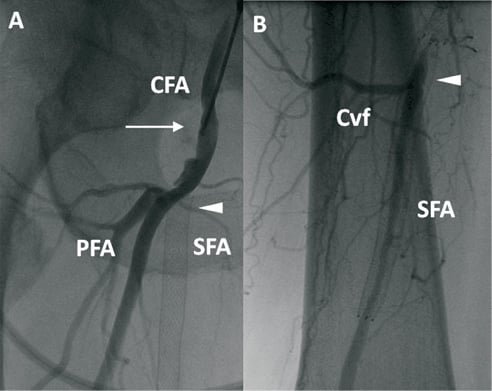
Figure 1. Baseline angiography. A) Selective angiography through right common femoral antegrade vascular access (arrow) showing restenotic occlusion at the proximal edge of previously implanted stent (arrow head). CFA: common femoral artery; SFA: superficial femoral artery: PFA: profunda femoral artery. B) Distal reperfusion (arrow head). Cvf: Collateral vessels flow. SFA: superficial femoral artery
THE INVITED EXPERTS’ OPINION
Over the last five years randomised trials1,2 have favoured stent implantation as the method of choice in patients with arteriosclerotic lesions of the superficial femoral artery. With the growing experience of investigators and a continuous development of stent technology the complication rate is low3 and results have continuously improved.
However, with stenting being increasingly performed in the SFA, a growing number of patients will require repeat treatment for in-stent restenosis3,4. In particular, for long stented segments the rate of in-stent restenosis is high3.
Currently, there is no consensus as to how best to treat SFA in-stent restenosis. The Trans-Atlantic Inter-Society Consensus II guidelines5 do not offer recommendations for the treatment of restenoses after stent implantation.
At present the available data on the optimal treatment strategy are limited and the associated outcome is unknown6,7.
In 2008 the effectiveness of drug-coated balloons compared to standard balloons for the treatment of the SFA was demonstrated. The use of paclitaxel-coated balloons significantly lowered the incidence of restenosis at six months and the rate of target lesion revascularisation at six, 12, and 24 months8.
Our patient presented a complete in-stent restenosis of the SFA six months after stent implantation. The occluded segment is longer than 30 cm.
There are different therapeutic options in this case.
For a patient just with lifestyle-limiting claudication, a surgical repair, e.g., a fem-pop bypass, with significant perioperative mortality, cannot be justified.
By experience, we know that a standard balloon angioplasty yields poor results in such pathologies. The re-re-occlusion rate is extremely high.
Another option is a stent-in-stent implantation after recanalisation by balloon dilatation. This technique should also be avoided because it doubles the radial force to the vessel wall by two layers of stents in combination with an extensive amount of metal inside the artery. A failed concept cannot be improved by itself.
In our opinion, the implantation of covered stents, the so-called endoluminal bypass grafting, carries additional risks. The endograft, e.g., Hemobahn, would occlude the distal collateral coming from the deep femoral artery. In case of a re-occlusion of the graft, a collateral flow is impossible, inducing an acute ischaemic syndrome of the lower leg.
From our experience the optimal strategy is currently to perform a standard recanalisation procedure and to use drug-coated balloons to inhibit recurrent growth of neointimal hyperplasia. This treatment modality is already proven in coronary arteries9.
Just recently, a small prospective registry has demonstrated the potential beneficial effect of drug-coated balloons for the treatment of superficial artery in-stent restenosis10.
To prove this concept further, we have conducted a randomised multicentre trial (FAIR-Study), which compares the application of prolonged standard balloon dilatation versus the use of drug-coated balloons for the treatment of SFA in-stent restenosis (Clinical trial.gov#: NCT01305070). Enrolment has been completed and the final data will be presented this year.
Conflict of interest statement
H. Krankenberg receives research support from Medtronic. T. Zeller receives study grants from Medtronic, Biotronik, C.R. Bard (Lutonix), Cook Medical and Eurocor.
THE INVITED EXPERTS’ OPINION
The patient suffers from early re-occlusion of the superficial femoral artery which occurred only six months after treatment with a SMART stent (Cordis, Johnson & Johnson, Warren, NJ, USA). We would first consider an endovascular treatment with drug-eluting technology, for example with a Zilver® PTX™ drug-eluting stent (Cook Medical, Bloomington, IN, USA). The notable advantages of drug-eluting stents are manifested in treating in-stent restenosis and long lesions1. In a subanalysis of the Zilver® PTX™ trial, a total of 142 lesions were lesions with in-stent restenosis. The freedom from target lesion revascularisation was 84% after 12 months. The primary patency rate was 80% after 12 months for the in-stent restenosis lesions. These results imply that stenting with the drug-eluting Zilver PTX stent is an effective treatment of in-stent restenosis.
An alternative would be PTA with a drug-coated balloon (DCB), but for the moment no published data are available to support this.
However, because the patient underwent several percutaneous coronary revascularisations for drug-eluting stent in-stent restenosis, drug-eluting technology does not seem to work well for this patient. This brings us to the decision to perform a femoropopliteal above the knee bypass with PTFE graft. We do not opt for a reversed vein bypass because the patient’s vena saphena might be needed in the future for CABG or a more distal revascularisation of the leg.
We will discharge the patient on antiplatelet therapy, meaning a 100 mg of Cardioaspirin daily for life, 75 mg of clopidogrel daily for three months and subcutaneous low molecular weight heparin for three weeks postoperatively.
Conflict of interest statement
The authors have no conflicts of interest to declare.
ACTUAL TREATMENT AND MANAGEMENT OF THE CASE
Before starting antegrade percutaneous intervention, the patient was screened for enrolment into the Intravascular Stenting and Angiographic Results: Randomized Comparison of Paclitaxel-Eluting Balloon vs. Conventional Balloon for In-Stent Restenosis of the Superficial Femoral Artery in patients with symptomatic peripheral artery disease (ISAR-PEBIS) trial.
After obtaining informed consent from the patient, he was randomly allocated to angioplasty with PEB. The patient was pretreated with a loading dose of 600 mg clopidogrel two hours prior to the procedure. After administration of 5,000 IU heparin intravenously, a 4 Fr Berenstein catheter (Cordis, Johnson & Johnson, Warren, NJ, USA) and a 0.014-inch ASAHI Confianza Pro 9 guidewire (Abbott Vascular, Abbott Park, IL, USA) were selected (Figure 2A). The attempt to cross the occlusion was successful and the guidewire was placed in the tibial vessels. Next, a conventional, uncoated, over-the-wire PTA balloon catheter (6.0×120 mm, Fox sv; Abbott Vascular) was inflated at stenotic sites (6-12 atm, 2 minutes) allowing straight vessel flow restoration, in the presence of several residual intra-stent stenoses. At this point, the 0.014-inch guidewire was exchanged for a standard 0.035-inch guidewire to provide more support during subsequent PEB advancement (Figure 2B). Appropriate PEB size and length were selected taking as a reference a ruler placed behind the patient’s leg. Accordingly, three IN.PACT Admiral Paclitaxel-Eluting Balloon catheters (6.0×120 mm; Medtronic Inc., Frauenfeld, Switzerland) were used (12 atm, 3 minutes; Figure 2B), in line with the “one-time inflation” rule and with the intention of covering 10 mm distal and proximal to previously stented segments (Figure 2C).
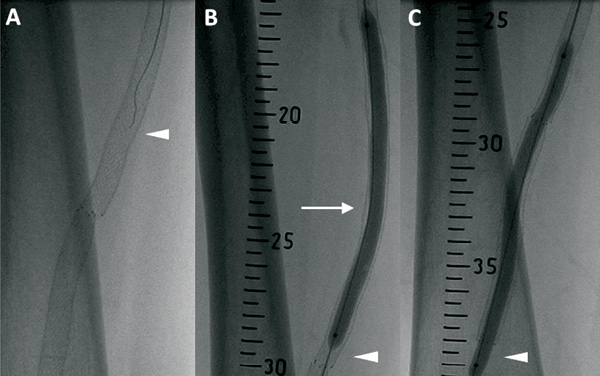
Figure 2. lntervention. A) The 0.014-inch guidewire (ASAHI Confianza Pro 9. Abbott Vascular, Abbott Park, IL, USA) attempting to cross the occlusion (arrow head). B) The 0.035-inch guidewire (arrow head) used to support paclitaxel-eluting balloon (IN.PACT Admiral. Medtronic Inc., Frauenfeld. Switzerland) angioplasty (arrow head). C) Angioplasty was performed by covering 10 mm distal and proximal to previously stented segments (arrow head).
Final angiography confirmed an optimal result, with complete vessel patency to the level of the foot vessels (Figure 3A-D). The puncture site was managed with manual compression after heparin effect reversal with protamine administration. Post-procedural course was uneventful and the patient was discharged on the following day in good clinical condition.
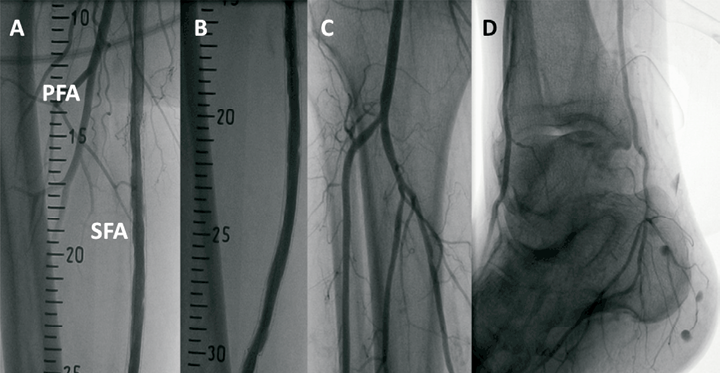
Figure 3. Final anglography. A-B) Optimal in-stent result. SFA: superficial femoral artery; PFA: profunda femoral artery. C) Leg vessel patency. D) Complete foot vessel visualisation.
Six-month surveillance angiography documented persistent vessel patency in the treated segments with excellent antegrade flow, in the absence of significant in-stent restenosis or de novo lesions (Figure 4A-C). Moreover, the duplex examination evidenced good Ankle-Brachial Index (ABI) (right side: 0.92; left side: 1.1). At 12-month follow-up, the patient remained asymptomatic and underwent ultrasonography and Doppler studies (ABI right side: 0.86; left side: 1.1) as well as computed tomography scan evaluation: in both cases, vessel patency was confirmed in the presence of good flow haemodynamics, to the level of the distal vessels (Figure 5A-B).
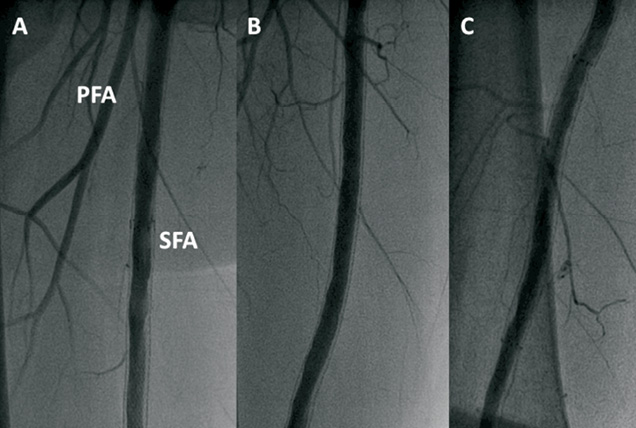
Figure 4. Six-month surveillance angiography. A-C) Persistent vessel patency in the treated segments in the absence of significant in-stent restenosis is observed. SFA: superficial femoral artery; PFA: profunda femoral artery
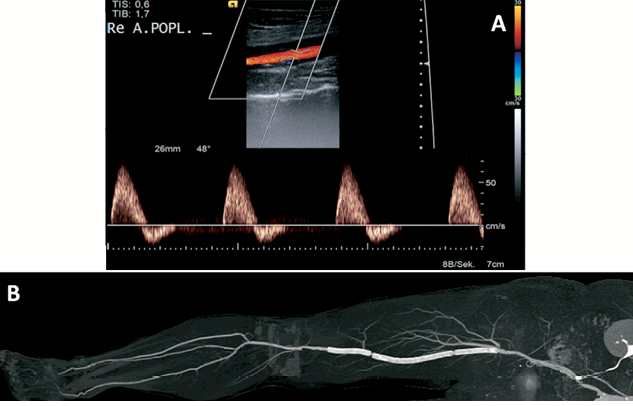
Figure 5. Twelve-month follow-up. A) Colour-flow and duplex scan of the affected limb documenting acceptable flow distal to the treated segment. Re A.POPL.: right popliteal artery. B) Computed tomography scan showing vessel patency in the affected limb up to the pedal vessels.
In patients complaining of peripheral artery disease (PAD), the SFA represents a common location for obstructive atherosclerotic disease2. Percutaneous intervention with conventional balloons and stents provided new options for the treatment of this arterial segment. However, even though a successful acute outcome can be achieved in more than 95% of cases, results are hampered by low durability of efficacy and high rates of restenosis3,4. In this regard, the SFA remains a “bad conduit”, prone to re-occlusions, even if stent use reduces the impact of early elastic recoil and flow-limiting dissections observed in the aftermath of plain balloon angioplasty5.
To date, prevention and therapy of restenosis after angioplasty or stenting have been predicated on modalities that can deliver sustained antiproliferative drug release into the vessel wall. This has led to the introduction of specifically-designed drug-eluting stents (DES) for use in PAD settings6. However, concerns persist in relation to the role of stent therapy in femoropopliteal disease, and this has focused attention on the development of non-stent-based methods of achieving local drug delivery in lower limb interventions7.
In vitro and in vivo models showed that, during angioplasty with a drug-eluting balloon, contact between vascular smooth muscle cells and paclitaxel, a highly lipophilic anti-neoplastic drug, was sufficient to provide long-lasting cell proliferation inhibition8.
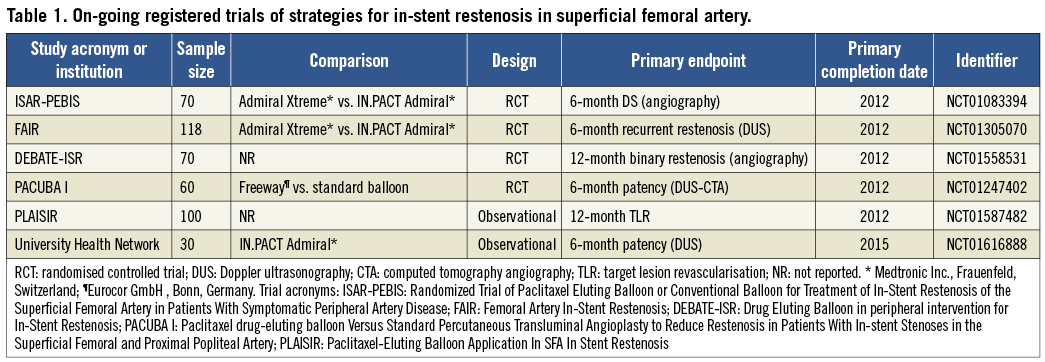
Subsequent investigations, focusing on the antirestenotic efficacy of different paclitaxel formulations in lower extremity vascular disease, demonstrated that therapy with PEB is superior to an approach using paclitaxel-containing contrast medium9. Recently, the antirestenotic efficacy of PEB was found superior to plain balloon angioplasty for SFA disease10. Moreover, data relating to PEB efficacy in coronary in-stent restenosis are promising11. However, its role in the treatment of in-stent restenosis in SFA lesions remains largely unstudied. Indeed, there may be important differences in device performance between the coronary and peripheral vascular disease beds. In this respect, the performance of DES may be a case in point with the excellent results obtained in the coronary arena not unequivocally replicated in SFA disease5,12,13. Moreover, literature addressing DES or PEB performance in SFA restenotic lesions is scarce14,15. Against this background the ISAR-PEBIS trial and a number of other trials are currently enrolling patients undergoing PEB vs. standard balloon angioplasty for in-stent restenosis of the SFA (Table 1).
The ISAR-PEBIS trial is an on-going, investigator-initiated, randomised clinical trial designed to compare the efficacy of PEB (IN.PACT Admiral Paclitaxel-Eluting Balloon catheter; Medtronic) vs. conventional balloon angioplasty (Admiral Xtreme Balloon catheter; Medtronic) for the treatment of SFA in-stent restenosis. This trial is powered for a primary angiographic endpoint (percentage of diameter stenosis at six-month follow-up), with a total of 70 patients to be enrolled. Patients with symptoms of lifestyle-altering claudication or rest pain with or without tissue loss (Rutherford Class 2-6) and SFA in-stent restenotic (≥70% of vessel diameter) or occlusive lesions are eligible for inclusion. The key exclusion criteria are the presence of poor inflow due to untreated ipsilateral aorto-iliac lesion (≥70%), popliteal atherosclerotic disease with stenosis (≥70%), the absence of ≥1 vessel run-off in below-the-knee arteries and thrombotic vessel occlusion with acute symptoms onset. Patients will be assigned to interventions in a random 1:1 fashion. Provisional stenting after angioplasty is allowed at the discretion of the operator. Dual antiplatelet therapy will be prescribed up to three months, with aspirin to be continued indefinitely. Six-month surveillance angiography is scheduled for all patients. Clinical follow-up and ABI, walking distance, duplex sonography or other non-invasive imaging (i.e., computed tomography scan) will be planned at six, 12 and 24 months after the index procedure. The trial is registered at www.clinicaltrials.gov (NCT01083394).
In the current report we present a case of angioplasty with PEB to treat a 67-year-old, diabetic male complaining of symptomatic long-segment in-stent restenosis of the SFA. Six-month angiography as well as 12-month non-invasive imaging confirmed very good outcome in the absence of significant disease.
Although a promising therapy, the specific place of PEB therapy in the management of SFA in-stent restenosis awaits the availability of data from specifically-designed randomised clinical trials. In this respect, it is hoped that results from ISAR-PEBIS and other randomised trials, which are comparing the efficacy of conventional PEB angioplasty vs. uncoated balloon for the treatment of SFA in-stent restenosis, will shed more light on the best treatment strategy for these complex patients.
Conflict of interest statement
The authors have no conflicts of interest to declare.

