
Abstract
Aims: The aims of this study were to assess the incidence and predictors of superficial femoral artery (SFA) stent thrombosis (ST) in a large patient cohort.
Methods and results: A total of 984 stented SFA lesions were retrospectively analysed in 717 patients. We observed an overall ST rate of 7.5% (74/984): 14% occurred early within 30 days after stenting, 51% during the first year thereafter and 35% later than one year. The estimated five-year probability of ST was 13.4% (95% confidence interval [CI]: 10.0% to 16.7%). Significant predictors of ST were stent length (hazard ratio [HR] 1.09, 95% CI: 1.06 to 1.11, p<0.001), lesion length (HR 1.10, 95% CI: 1.08 to 1.13, p<0.001), female gender (HR 1.79, 95% CI: 1.12 to 2.86, p=0.015), chronic total occlusion (CTO) (HR 4.21, 95% CI: 2.51 to 7.05, p<0.001), implantation of more than one stent (two stents: HR 6.06, 95% CI: 3.35 to 11.0, p<0.001; three or more stents: HR 16.83, 95% CI: 9.43 to 30.0, p<0.001) as well as lesion complexity criteria as expressed by TASC II C/D (HR 17.7, 95% CI: 5.56 to 56.1, p<0.001).
Conclusions: ST after SFA stenting was a common adverse event in our cohort and peaked during the first year after stent implantation. Independent predictors of ST included lesion length and stent length, female gender, presence of CTO, number of implanted stents and lesion complexity.
Introduction
Atherosclerotic peripheral artery disease (PAD) affects more than 200 million people worldwide1. Current guidelines give precedence to endovascular approaches in less complex Trans-Atlantic Inter-Society Consensus (TASC) A and B lesions but also support its use in more complex TASC C and D lesions in centres with dedicated interventional experience2. In this context, self-expanding stents are well established and widely used for primary stent implantation in symptomatic PAD because of favourable results in shorter lesions (<150 mm) of the superficial femoral artery (SFA) or as a bail-out procedure in case of a flow-limiting dissection, early elastic recoil or significant residual stenosis after balloon angioplasty3. Although results have improved over time with one-year primary patency rates ranging up to 70% for stents of the latest generation4, target lesion failure (TLF) with thrombotic occlusion of the SFA still represents a major concern associated with acute limb ischaemia and the risk of subsequent amputation.
Although there is substantial evidence regarding in-stent restenosis (ISR), with a wide range of studies reporting long-term patency rates and clinical outcome, dedicated studies focusing on the description of ST occurrence are still scarce5,6,7,8. In this study, we aimed to analyse the incidence and predictors of ST after SFA stenting in a large patient cohort over a period of one decade.
Methods
PATIENT POPULATION
Between January 2008 and December 2018, 717 patients with symptomatic lower extremity PAD and endovascular SFA stenting were retrospectively identified and analysed at both sites of our institution. Patients with endovascular therapy of other arterial vessels except the SFA or without stent implantation were excluded from the analysis. More than one third of all patients (37.2%; 267/717) underwent stenting of both SFA usually performed in staged procedures.
PROCEDURAL ASPECTS
The decision regarding endovascular revascularisation at our institution was individually made in every patient based on the existence of relevant clinical symptoms and the presence of angiographically verified diameter stenosis at least 50% or occlusion. All procedural considerations were left to the discretion of the interventionalist. The number of below-the-knee (BTK) run-off vessels was angiographically assessed for all patients during the index procedure. All patients were routinely prescribed life-long low-dose aspirin (100 mg/d), which was complemented with a loading dose of 600 mg clopidogrel followed by 75 mg/d for four weeks in patients with uncomplicated first-time stenting in shorter lesions. In the case of longer stents, usage of multiple stents in the SFA or in the case of eluting devices (drug-coated balloons [DCB]/drug-eluting stents [DES]) dual antiplatelet therapy (DAPT) was prescribed for three to six months. Duplex ultrasound and ankle brachial index were assessed in all patients prior to the endovascular procedure and before discharge. Written informed consent was obtained from all patients prior to the endovascular procedure. Approval for data analysis was obtained from the local ethics committee.
FOLLOW-UP
Follow-up visits were routinely scheduled after 3, 6 and 12 months, as well as annually, after the index procedure. In case of symptoms, medical consultations in between these scheduled appointments were possible at any time. The clinical, demographic and, if available, angiographic data were obtained from the charts and reports of the outpatient department and were supplemented by data from follow-up telephone calls. ST was assumed in the case of acute onset (<2 weeks) of claudication and/or signs of critical limb ischaemia (CLI) in combination with ultrasound angiographic evidence as well as evidence of occlusive thrombus formation within the stented segment (±5 mm proximal and distal to a stent edge) of the SFA. Accounting for the temporal occurrence of ST, we refer to the definitions of the Academic Research Consortium (ARC), which established the definition of ST in coronary arteries9. Based upon those definitions, we subdivided the occurrence of ST into acute (<24 hrs), subacute (24 hrs to 30 days), late (31 days to one year) and very late (>1 year) ST.
STATISTICAL ANALYSIS
A statistical analysis is provided in Supplementary Appendix 1.
Results
PATIENT CHARACTERISTICS
A total of 984 endovascular stent procedures were performed in 717 patients with de novo SFA lesions. More than half of the population was male (65%; n=640). Mean patient age was 70.8±9.6 years. The population exhibits a typical cardiovascular risk profile and cardiovascular comorbidities with one third diabetics and about two thirds having concomitant coronary artery disease (Table 1). The majority of patients (74%) had a stable Rutherford category 3 claudication while the remaining patients presented with CLI (Rutherford category 4-6). Median follow-up time was 13 months.
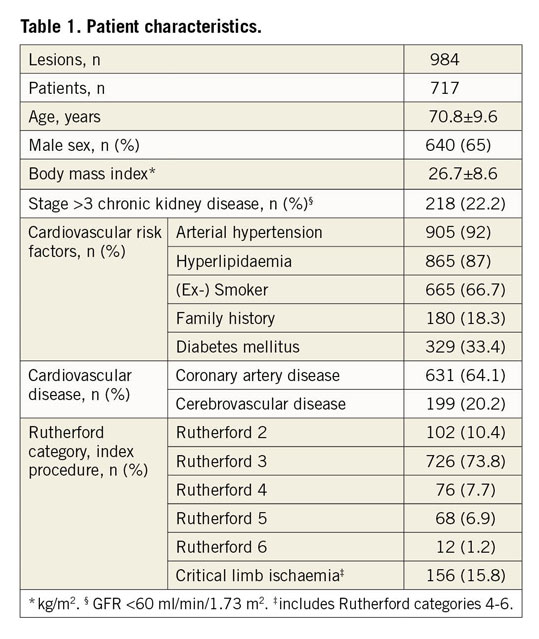
PROCEDURAL CHARACTERISTICS
Procedural characteristics are shown in Table 2. More than one third (38.8%) of the index lesions were chronic total occlusions (CTO) and more than half of the patients (57%) were classified as TASC II C and D with mostly moderate calcification. The vast majority of lesions (99.7%) were treated with nitinol self-expanding stents (53% S.M.A.R.T.® Control™ [Cordis, Cardinal Health, Milpitas, CA, USA]; 40% EverFlex™ [Medtronic, Minneapolis, MN, USA]; <1% each: Zilver® PTX® [Cook Medical, Bloomington, IN, USA]; Absolute Pro® [Abbott Vascular, Santa Clara, CA, USA]; sinus-SuperFlex® [Optimed, Ettlingen, Germany]; Maris Plus® [Invatec, Roncadelle, Italy]; GORE® VIABAHN® [W.L. Gore & Associates, Inc., Flagstaff, AZ, USA]). Three lesions (0.3%) were treated with a balloon-expandable stent (Omnilink Elite® [Abbott Vascular]). In total, 1,264 stents were implanted in 984 lesions, which accounts for an average of 1.28±0.6 stents per lesion. The mean stent diameter and length were 6.8±0.84 mm and 151±95.6 mm, respectively. Most patients had a two- or three-vessel BTK run-off (43.4% and 37.3%, respectively).
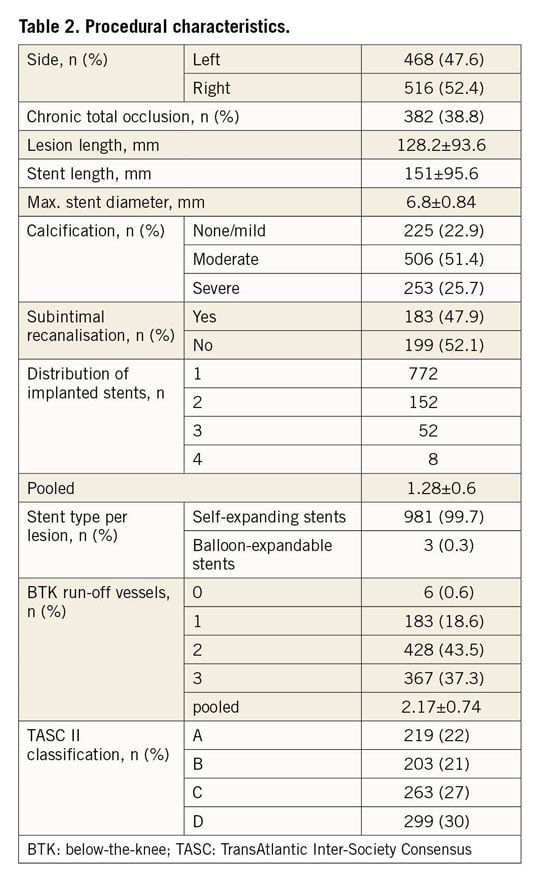
STENT THROMBOSIS
Characteristics related to the thrombotic events are shown in Table 3. Overall, thrombotic stent occlusion occurred in 74 endovascular procedures, resulting in an ST rate of 7.5% in the entire cohort. However, the estimated five-year probability of ST was 13.4% (95% confidence interval [CI]: 10.0% to 16.7%). Six patients suffered from ST of both limbs at different times. Mean and median duration from index procedure to the occurrence of ST was 13±15 and 7 (0-86) months, respectively. Based on event timing, 14% of patients had an acute or subacute ST during the first 30 days after stent implantation. Half of the patients (51%) developed a late ST between day 31 and one year and 35% after the first year. Clinically, one third of all ST patients (33.8%) presented with a CLI while the majority had a Rutherford stage 3 claudication (63.5%). No ST was detected within a lesion treated with a balloon-expandable stent.
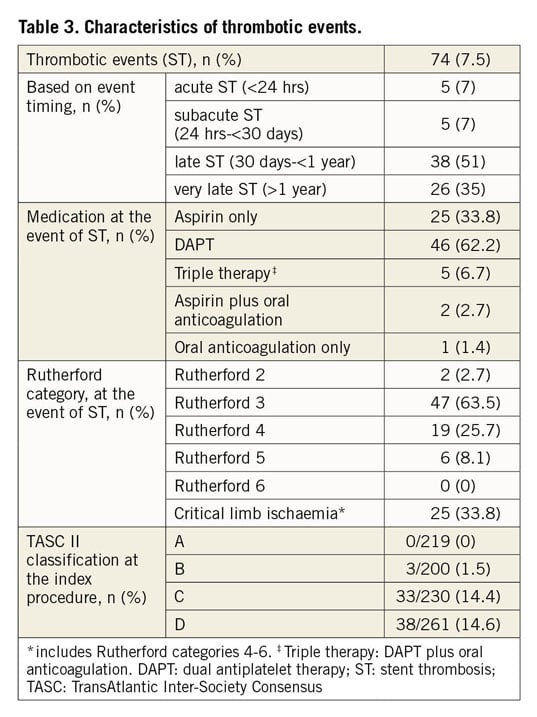
Median duration of DAPT in patients with ST was 3 (0-12) months after the index procedure. At the time of ST, 33.8% of our patients were on a single antiplatelet therapy with aspirin only and one patient (1.4%) received oral anticoagulation (OAC) only. However, the majority of patients received combination therapy: 55.4% were on DAPT only, 6.7% had a therapy with OAC and DAPT, and 2.7% were on a combined therapy consisting of OAC and aspirin, respectively.
TREATMENT OF ST
Thrombotic stent occlusion was primarily treated using an endovascular approach. In order to reduce thrombus burden, initial thrombus aspiration was performed for more than half of the thrombotic events (59.5%). This was achieved either by manual catheter-based thromboaspiration in nine cases (12.2%) during the early years of observation or most commonly since the year 2012 by mechanical rotational thrombectomy using the Rotarex® device (Straub Medical, Wangs, Switzerland) in 35 cases (47.3%). Catheter-directed thrombolysis (CDT) was performed in two (2.7%) cases.
After thrombus extraction, plain old balloon angioplasty or DCB only were used in 14.9% and 25.7% of thrombotic stent occlusions, respectively. In more than half of the ST lesions (56.7%), the interventional strategy was angioplasty followed by stent implantation in order to attach residual thrombus burden. Of these, 21.6% were additionally treated with a DCB. Intravascular imaging modalities were not systematically applied. Distal embolisation occurred in 18 out of 74 cases (32.1%) and was treated by simple ballooning in more distal vessels of the lower leg or usage of the Rotarex device in proximal lesions of the lower leg. Two patients (2.7%) underwent urgent bypass surgery due to unsuccessful endovascular recanalisation.
PREDICTORS OF ST
A variety of factors was analysed in order to identify predictors for the occurrence of ST in the SFA (Table 4). Significant independent predictors were the number of implanted stents (p<0.001), lesion and stent length (p<0.001) as well as the presence of a CTO at the index procedure (p<0.001) and lesion complexity as expressed by TASC II class C and D (p<0.001). Additionally, female gender was associated with the occurrence of ST (p=0.015) (Figure 1, Figure 2, Supplementary Figure 1, Supplementary Figure 2).
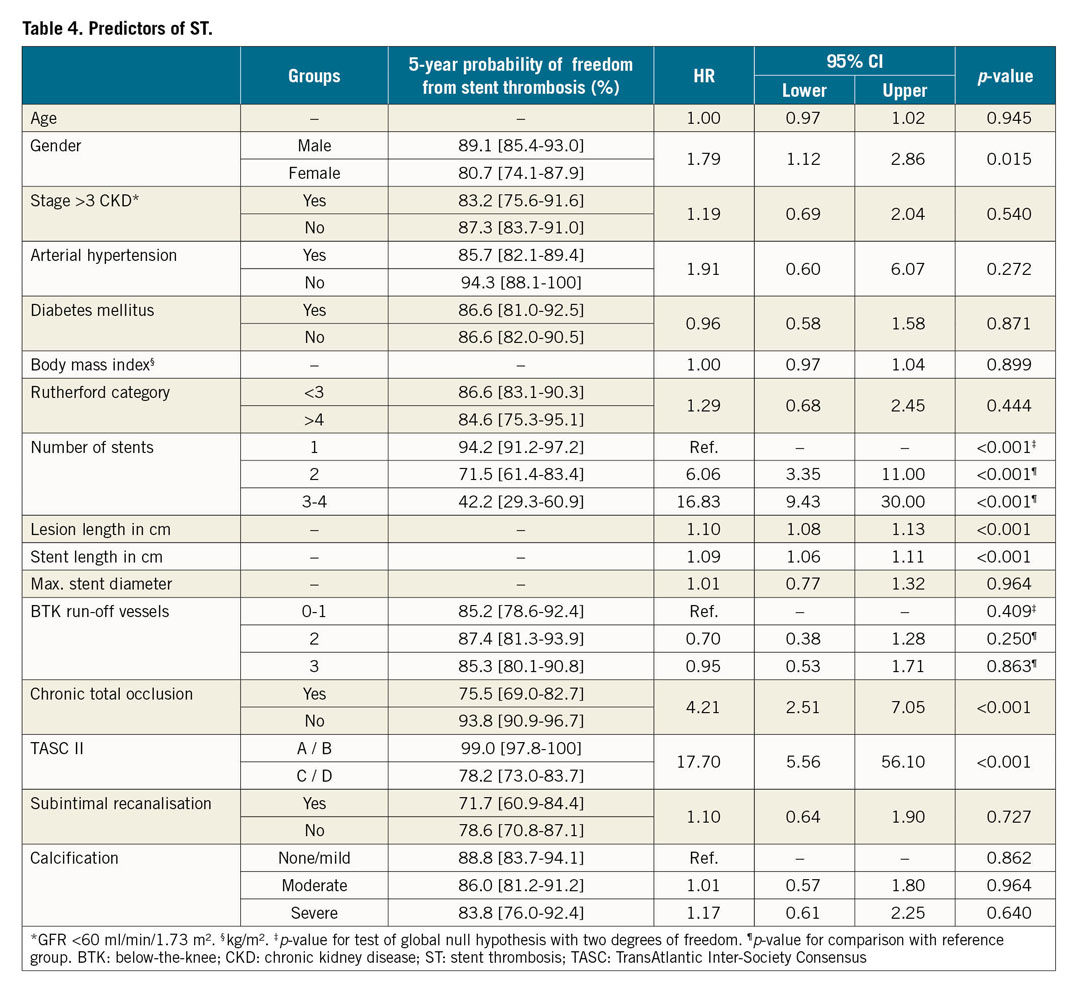
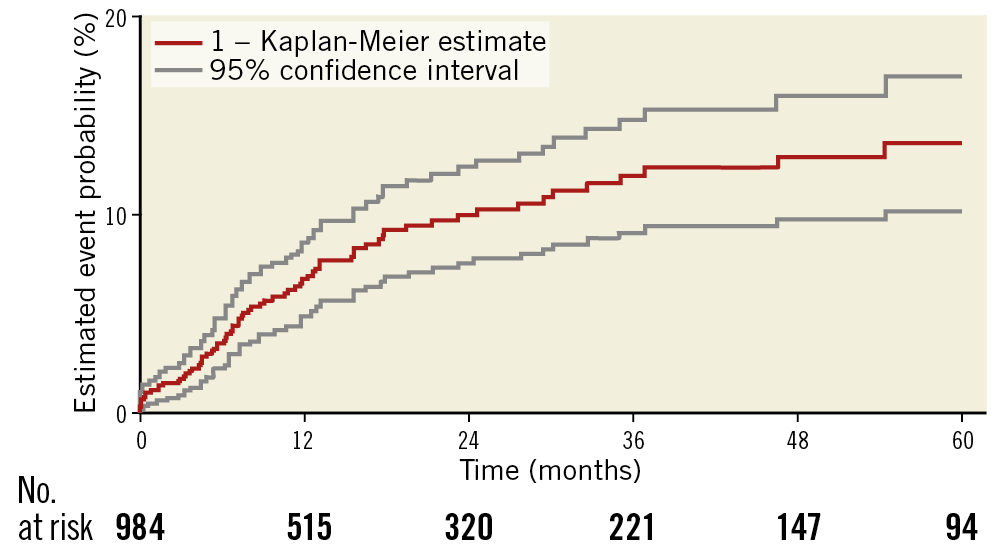
Figure 1. Kaplan-Meier survival curves showing the rate of ST. Overall five-year freedom from ST. ST: stent thrombosis
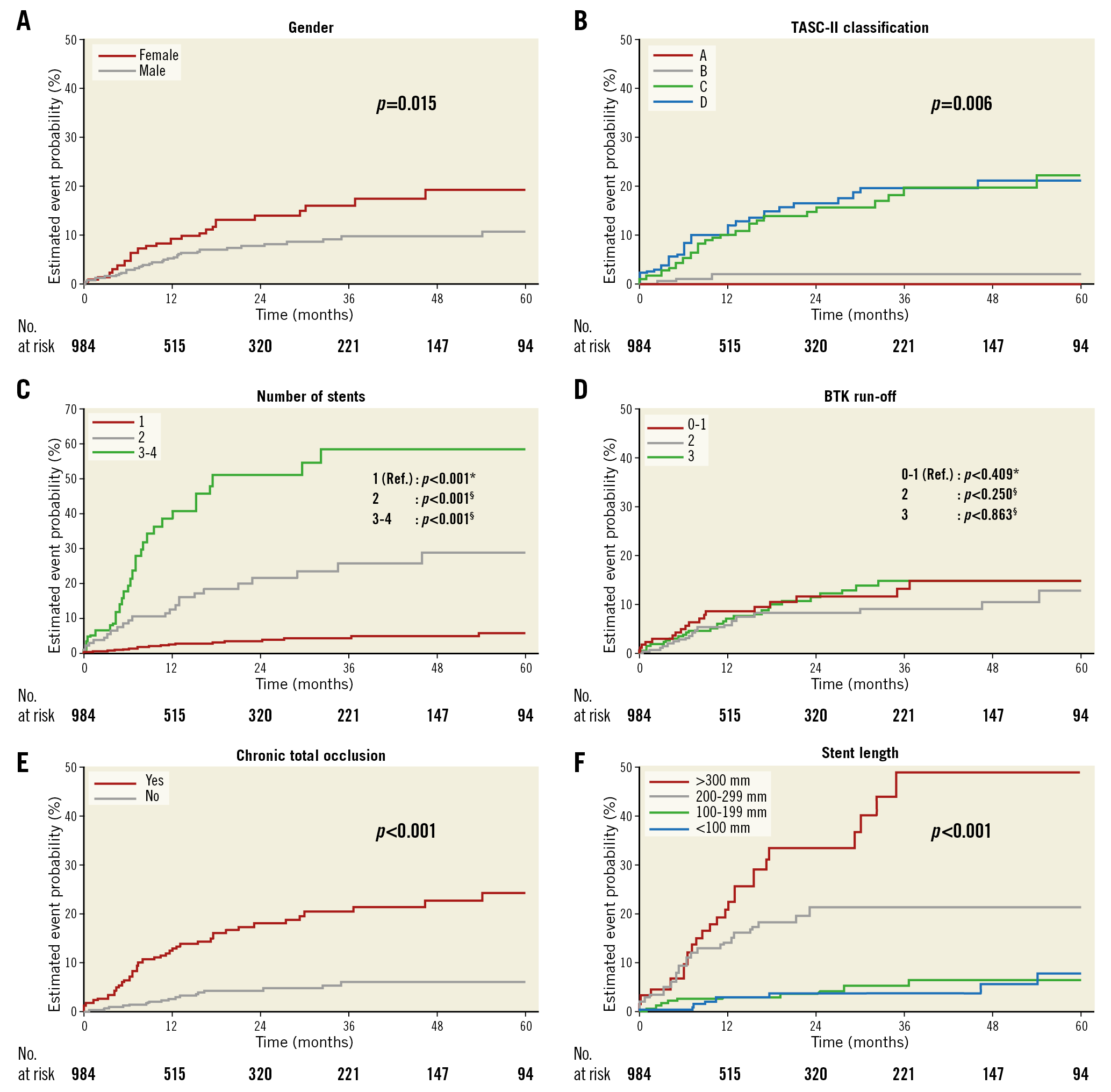
Figure 2. Five-year freedom from ST according to pre-specified subgroups. Kaplan-Meier survival curves showing the rates of ST for gender (A), TASC II classification (B), number of stents (C), BTK run-off (D), CTO (E), and stent length (F). *p-value for test of global null hypothesis with two degrees of freedom; §p-value for comparison with reference group. BTK: below-the-knee; CTO: chronic total occlusion; ST: stent thrombosis; TASC: TransAtlantic Inter-Society Consensus
Discussion
The major findings of this analysis of a large cohort of patients undergoing SFA stenting were: 1) the overall rate of ST was high and averaged 7.5% with an estimated five-year probability of ST of 13.4% while the majority of ST occurred during the first year after stent implantation, and 2) the development of SFA ST was significantly associated with longer lesion and stent length, female gender, stenting of chronic total occlusion, increasing number of implanted stents as well as with high complexity of the underlying lesion as expressed by TASC II class C and D.
INCIDENCE AND TIMING OF STENT THROMBOSIS
The rate of ISR ranges from 9% up to 37% after 12 months in randomised studies and meta-analyses of controlled and uncontrolled trials3,4,10. However, in contrast to the existing body of knowledge about ISR, the mechanisms leading to infra-inguinal ST have hardly been studied to date. Thrombotic stent occlusion rates in the SFA were previously reported as ranging from 2 to 22% in a small number of studies without detailed appreciation of lesion complexity according to current TASC II classifications6,7,8,10,11,12. In those studies, more than 50% of patients were stented for CTO of the SFA and there was a wide range of lesion and stent lengths among the studies. However, to the best of our knowledge, only the studies by Banerjee et al and Katsanos et al were designed to estimate the development of lower extremity ST with an observed prevalence of 4.3 and 6.1%, respectively7,8.
The ST rate according to our observation continued to increase up to year 5 after the intervention with an estimated five-year probability of 13.4% (95% CI: 10.0% to 16.7%). Similar findings were reported by Banerjee et al and Katsanos et al7,8. However, the median follow-up in our cohort was 13 months and considerably longer than that reported in other studies. Thus, the development of very late ST may have been detected more frequently in our patients, which might possibly explain the slightly higher rate of thrombotic occlusions in our population.
POST-INTERVENTIONAL MEDICATION
Premature discontinuation of DAPT is a well-known major risk factor for the occurrence of ST after stent implantation in the coronary vasculature13. According to current guidelines, DAPT after peripheral stenting is recommended for at least four weeks, which might be extended in case of the use of drug-coated devices2. However, the evidence for the post-interventional antiplatelet treatment in PAD is very poor and high-quality data from adequately powered randomised trials are lacking14. The ZEPHYR investigators, evaluating the Zilver PTX stent for femoropopliteal lesions, discussed that interruption of DAPT after peripheral DES implantation markedly increased the risk for ST in their population, averaging 2%10. In contrast, Dohi et al failed to show sufficient effectiveness of antiplatelet therapy or warfarin for the prevention of in-stent occlusion in their large cohort of more than 2,000 patients6.
In our population, the majority of patients with ST received DAPT or were on an OAC treatment regime either exclusively or in combination with aspirin and/or clopidogrel at the time of the thrombotic event.
Recent data from a large subgroup analysis of the COMPASS trial including 7,470 patients with stable PAD showed that a combination of low-dose rivaroxaban and aspirin resulted in a significant reduction of cardiac (MACE) and major adverse limb events (MALE) compared with the standard treatment of aspirin alone15. Whether patients with symptomatic lower-extremity PAD undergoing endovascular revascularisation with stent implantation may benefit from a combination of antiplatelet therapy with direct oral anticoagulants in the post-interventional phase has still to be clarified. This notion is addressed by the VOYAGER-PAD trial (NCT02504216) comparing antiplatelet therapy and low-dose rivaroxaban with antiplatelet alone in over 6,500 PAD patients after endovascular or surgical limb revascularisation which is expected to be completed in 2019 and which will provide further evidence with respect to novel medical therapy approaches16.
PREDICTORS OF STENT THROMBOSIS
As mentioned above, various parameters were significantly associated with the development of ST in our cohort. Only limited data from registries describing predictors for ST after SFA stenting are available to date. In the study of Katsanos et al, CLI and the implantation of covered nitinol stents could be identified as the only predictors for ST8. On the other hand, Banerjee et al identified stent implantation in the setting of CTO and ISR lesions, longer lesion or stent length and also the implantation of covered stents as being predictive for ST7. At our institutions, covered stents are not routinely implanted in the SFA as a primary revascularisation approach and are solely used as a bail-out strategy in case of iatrogenic vessel perforation or in case of an aneurysm. Therefore, we cannot sufficiently assess the rate of ST related to the use of this type of stent. However, since both studies identified covered stent grafts as being associated with ST, it appears reasonable that the larger and more thrombogenic surfaces of these types of stent might bear a higher thrombogenic risk than uncovered stents.
Dohi et al observed that the patency rate after femoropopliteal interventions in patients with CLI was 50% lower than in claudicants6. In our cohort, ST was not related to the presence of CLI. However, CLI patients accounted for 16% of our population, which was less frequent when compared to the aforementioned studies which included 23% and 49% of patients with CLI, respectively7,8.
Although Banerjee et al observed that ST was more likely to occur in men than in women, we identified female gender as being significantly associated with the development of ST in our population. Reduced long-term patency rates in women were also reported by Pulli et al investigating gender-related outcomes after endovascular treatment of infra-inguinal PAD. Although they demonstrated a similar ST rate in men and women, poorer long-term patency rates were detected in women17. In line with these findings, Ihnat et al observed a non-significant trend for women to develop ST or significant ISR (37%) when compared to men (29%)11, and Dohi et al indicated a 75% higher in-stent occlusion rate in female patients in a registry enrolling patients with nitinol stents for femoropopliteal lesions6.
Also, the anatomic complexity of the lesion may be of importance for the outcome after endovascular revascularisation in terms of stent failure as well as for the development of ST. In line with our findings, previous studies have demonstrated that longer lesion and stent length such as TASC II class C and D lesions are prominent risk factors for the occurrence of ST, most likely caused by the large amount of foreign material combined with altered flow properties7,8.
Finally, patient-related factors such as pathological response to foreign stent material including the formation of ISR or neoatherosclerosis, which has been described by intravascular imaging techniques and seminal autopsy studies as a reason for ST development in coronary arteries, may play a similar role in the peripheral vasculature following stent implantation18. However, comparable data about intimal pathologies after stenting of the SFA which might allow a better understanding of the pathophysiology of peripheral artery ST and derivation of potential strategies for its prevention are still warranted.
Limitations
Due to the long observation period of more than one decade, treatment changes might have had an impact on study outcome, e.g., no uniform antithrombotic regimen or indication for revascularisation, the individual choice of stent type, and different experience of the performing interventionalists. The follow-up after 12 months included 52% of the patients; however, after five years, only 10% of the population has available follow-up data. Finally, we did not systematically use the latest intravascular imaging modalities in order to evaluate the underlying causes of ST further.
Conclusions
The occurrence of ST was a frequent adverse event affecting 7.5% of our patient population with an estimated five-year probability of 13.4%. ST occurred most commonly during the first year after stent implantation and was closely associated with longer stent and lesion length, female gender, stenting of chronic total occlusions, the usage of multiple stents and the presence of complex lesions according to TASC II class C and D.
|
Impact on daily practice Future studies are warranted to evaluate whether a closer follow-up, an intensification or prolongation of DAPT or anticoagulant therapy, or modifications of endovascular strategies might mitigate the incidence of stent thrombosis and translate into better patency rates after stenting of femoropopliteal PAD. |
Conflict of interest statement
The authors have no conflicts of interest to declare.

