Abstract
Aims: To examine the efficacy and durability of an interwoven self-expanding nitinol stent in the treatment of complex femoropopliteal artery lesions in unselected patients.
Methods and results: Five hundred and twenty-seven limbs in 470 patients with femoropopliteal arterial disease were treated with SUPERA stents. Follow-up data were prospectively collected in a single-centre registry and were available for 439 patients (492 limbs). The patients were followed by Doppler ultrasound, stent roentgenograms, estimation of Rutherford-Becker class (RBC) and ankle-brachial index (ABI). Total occlusions were present in 277 limbs (52.6%) and 52.4% had either moderate or severe calcification. The mean lesion length was 126.4 mm. The primary patency (PP) rates were 83.3% after 12 months and 72.8% at two years. The secondary patency rates were 98.1% after 12 months and 92.0% at two years. Patency rates did not differ between superficial femoral artery (SFA) and popliteal lesions. Between baseline and a mean of 21 months of follow-up, mean ABI increased from 0.53 to 0.91, and mean RBC decreased from 3.0 to 1.9 (p<0.001 for both comparisons). Radiographs performed on 229 patients at a mean of 16.6 months confirmed the absence of stent fractures in all patients.
Conclusions: Over a two-year surveillance period, the patency rate and fracture resistance of SUPERA stents implanted for complex femoropopliteal artery disease were high.
Introduction
Endovascular therapy is one option endorsed by current guidelines1,2 for the treatment of symptomatic femoropopliteal artery disease. However, data supporting specific therapies are still scarce.
There is some evidence that, particularly for non-focal lesions of the superficial femoral artery (SFA), patency rates after systematic primary stenting are significantly higher than after balloon dilatation and provisional stenting3. Based on the available clinical evidence4-6, implantation of nitinol stents into the femoropopliteal arteries has become a widely used technique overall resulting in improved clinical outcome of percutaneous procedures.
In contrast, there is still a relative uncertainty about the role of endovascular stenting in the popliteal artery and its efficacy in long lesions or calcified arterial segments. Particularly after treatment of long lesions, restenosis, kinking, compression and stent fractures remain an important concern. In the FESTO registry7, the incidence of stent fractures increased with lesion length, and major stent fractures were associated with restenosis. The same was observed in the DURABILITY I study4. In addition, a recently published 2012 article on the STELLA study for long lesions8 reported patency rates of 66% with a nearly 18% fracture rate, and the DURABILITY-200 study9 demonstrated a 65% patency rate with a 6% fracture rate.
The objective of this registry was to evaluate the efficacy and integrity of an interwoven vascular mimetic nitinol stent designed to withstand the compression, torsion, bending, lengthening and shortening found within the femoropopliteal segment. To derive both scientifically and clinically meaningful information for this “real-world” patient population, the registry intentionally allowed enrolment of a wide range of obstructions, including long lesions, total occlusions and highly calcified vessels, without pre-specified inclusion or exclusion criteria.
Methods
PATIENT POPULATION
Five hundred and twenty-seven limbs in 470 patients with femoropopliteal artery disease were treated with percutaneous transluminal angioplasty (PTA) followed by implantation of SUPERA stents (IDEV Technologies, Inc., Webster, TX, USA) in one vascular centre between June 2007 and October 2011. The decision to implant SUPERA stents was left to the operator’s judgement, although the general indications after primary PTA were followed, including haemodynamically severe residual stenosis, flow-limiting dissection or elastic recoil.
All patients underwent baseline physical examinations with a focus on manifestations of lower limb ischaemia, classified according to Rutherford and Becker10. Patients were asked for their pain-free walking distance while walking at a fast speed. According to our institutional routine, patients were classified to RBC 1 if walking distance was more than 300 m and to RBC 3 if walking distance was less than 100 m. RBC 2 was determined if walking distance was between 100 m and 300 m. In indecisive situations a treadmill test was performed.
The ABI was calculated and duplex ultrasound studies were performed, followed by angiography of the infrainguinal arteries to outline the vascular anatomy and define the lesion characteristics. The popliteal artery segments are defined as follows: P1 segment from intercondylar fossa to proximal edge of patella; P2 segment from proximal part of patella to centre of knee joint space; P3 segment (below knee popliteal artery) from centre of knee joint space to origin of anterior tibial artery. Degree of calcification was assessed routinely. It was graded on the basis of the amount of calcium deposits visible during fluoroscopy: a) mild for <5 mm calcium deposits in the vessel wall, b) moderate for >5 mm deposits, and c) severe for deposits filling up the entire vessel diameter.
In accordance with the institutional and local regulatory policies, all patients signed a written informed consent before undergoing the procedure.
INCLUSION AND EXCLUSION CRITERIA
There were no formal inclusion or exclusion criteria specified for this analysis other than that a SUPERA stent was implanted into the femoropopliteal segment to treat symptomatic de novo or restenotic lesions. Recipients of other stents during the index intervention were excluded from the analysis.
STENT PROCEDURE AND MEDICATION REGIMENS
The SUPERA stent system included a 7 Fr, and in the last years a 6 Fr coaxial delivery system with the pre-mounted stent. A detailed description of the system and of its deployment method has been published previously6. All lesions were predilated, and balloon size was determined by visual estimation. While post-dilatation was left to the discretion of the operator, predilatation was generally recommended. Additional devices were used at the investigator’s discretion: Rotarex (Straub Medical AG, Wangs, Switzerland) and thrombolysis were used in lesions with thrombotic material. The Outback device (Cordis, Johnson & Johnson, Warren, NJ, USA) was utilised if crossing the lesion was not possible by conventional means. Inflow and outflow lesions were treated if they were significant.
The antithrombotic regimen was administered according to the usual institutional practices. Periprocedural anticoagulation with ≥5,000 U of heparin was recommended. Dual antiplatelet therapy with clopidogrel and aspirin was administered for at least four weeks after the procedure, with the recommendation to continue aspirin indefinitely.
FOLLOW-UP AND STUDY ENDPOINTS
All patients were evaluated before their discharge from the hospital, and were scheduled to return for ambulatory follow-up visits according to our institutional standards at six and 12 months after the index procedure and every year thereafter for five years. The angiograms were analysed with regard to lesion properties (lesion location, lesion length, vessel diameter, degree of stenosis and calcification) by a single investigator (AP). At the follow-up visits, patients underwent physical examinations, estimates of RBC, ABI calculation and duplex ultrasound studies for the detection of restenoses. Not all patients were willing or able to return for the follow-up examinations (Figure 1): eight patients with eight treated limbs died before the first follow-up examination, and 23 patients (27 limbs) were lost to follow-up for unknown reasons. Duplex ultrasound and clinical assessment were available for 364 of the remaining 439 patients (412 limbs out of 492). In 75 patients, clinical assessment was performed by telephone contact. The duplex ultrasound recordings were analysed by an investigator who was not involved in the initial treatment procedure. A peak systolic velocity ratio ≥2.4 (corresponding to a ≥50% decrease in vessel diameter11) was used for the diagnosis of binary restenosis. To confirm the integrity of the stent, plain roentgenographic images of the stented segment were obtained in 229 patients (304 stents). This assessment was part of our routine follow-up protocol until 2010 and was typically performed at 12 to 24 months after the index procedure.
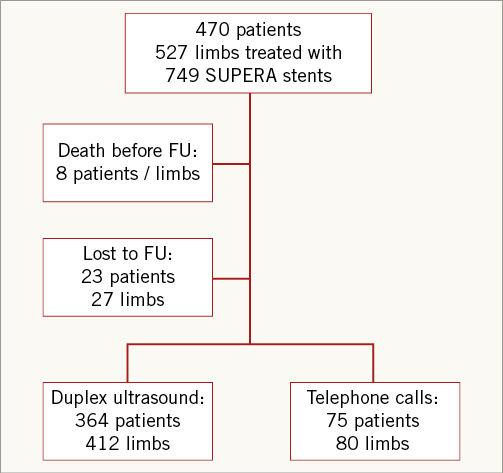
Figure 1. Study profile.
The primary efficacy endpoint of this analysis was primary stent patency (PP), defined as absence of binary restenosis or reocclusion on duplex ultrasound examination without repeat target lesion interventions. The secondary endpoints included procedural success, defined as a <30% residual vessel stenosis, clinical status according to the RBC, ABI measurements, overall survival, incidence of major and minor amputations, secondary patency, rate of target lesion revascularisation and incidence of stent fractures on radiographic screening.
Statistical analysis
Descriptive statistics were used to present: a) mean values and standard deviation (SD) for continuous variables, b) median values (range), and c) counts and percentages for categorical variables. Cumulative patency rates, freedom from target lesion revascularisation (TLR) and patient survival were estimated using Kaplan-Meier analyses. Between-group comparisons were made with the log-rank test. To delineate factors influencing primary patency Cox regression was performed on the following variables: age, gender, hypertension, diabetes mellitus, hyperlipidaemia, smoking, obesity, renal insufficiency, coronary artery disease, cerebrovascular disease, degree of stenosis, degree of calcification, lesion localisation, number of stents, lesion length, stent diameter, treatment for restenosis, treatment for in-stent stenosis, need for retrograde recanalisation and impaired infrapopliteal run-off. Mean ABI and RBC were compared at various time points, using the Student’s t-test for dependent samples. Statistical significance was defined as p<0.05. Analyses were performed using SPSS software, version 20 (IBM Corp., Armonk, NY, USA.).
Results
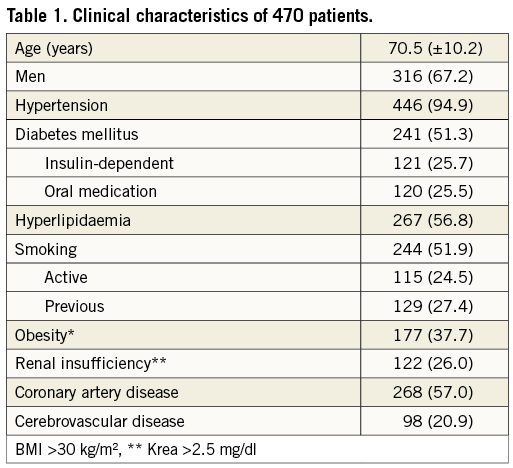
The analysis included 316 men (67.2%) and 154 women (32.8%), whose mean age was 70.5±10.2 years. Fifty-seven patients had bilateral treatment with SUPERA stents. The baseline clinical characteristics of these 470 patients are shown in Table 1. Cardiovascular risk factors were highly prevalent, including hypertension in 94.9%, current or former smoking in 51.9%, and diabetes in 51.3% of patients, while 53.9% of patients presented with severe claudication (RBC 3), 18.2% presented with critical limb ischaemia (RBC ≥4; Table 2), and 12.9% of patients had tissue loss (RBC 5). The mean ABI at rest before intervention was 0.53±0.14.
ANGIOGRAPHIC AND PROCEDURAL CHARACTERISTICS AND IMMEDIATE RESULTS
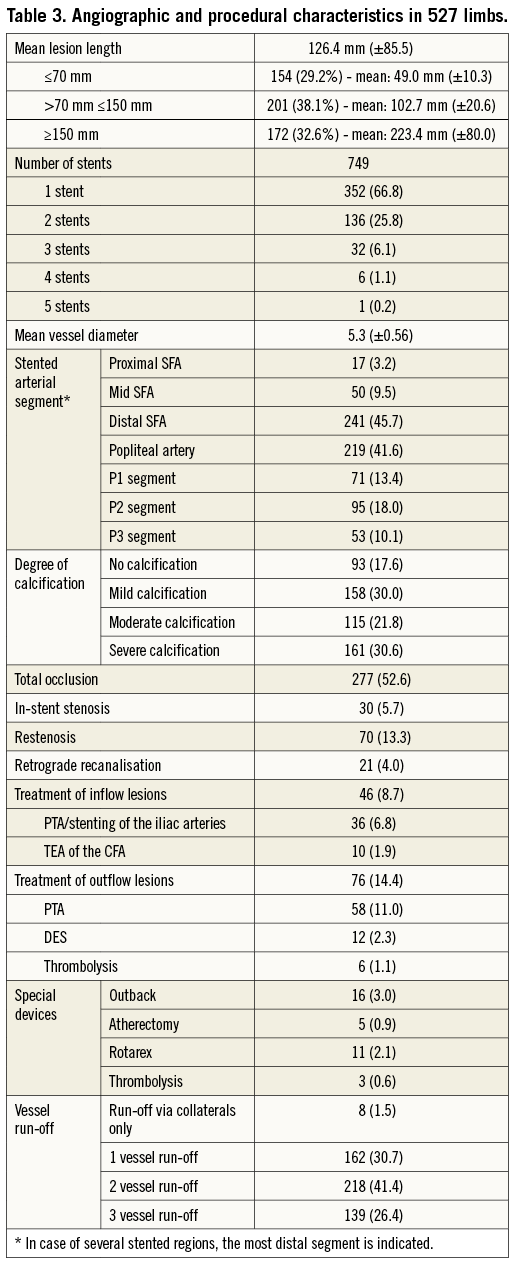
A total of 749 stents, 4 to 7 mm in diameter and 40 to 200 mm in length, were implanted in the 527 limbs. The treated lesion length ranged from 15 to 500 mm, representing a mean lesion length of 126.4±85.5 mm and a mean vessel diameter of 5.3±0.56 mm. The angiographic and procedural characteristics are shown in Table 3. Over 80% of the lesions were located in the distal SFA or popliteal segment, 52.4% of the lesions had either moderate (21.8%) or severe (30.6%) calcification, and total occlusions were present in the majority of the patients (52.6%).
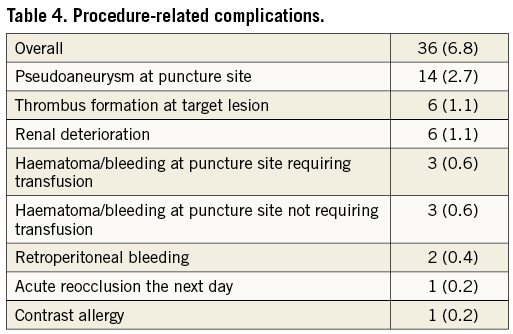
A visually estimated residual arterial stenosis <30% was achieved in 522 cases (99.1%). Procedure-related complications were recorded in 7.2% of the cases (Table 4).
CLINICAL FOLLOW-UP RESULTS
Clinical follow-up was conducted in 439 patients/492 limbs (93.4%) (Figure 1). The mean clinical follow-up time was 20.9±12.1 months. During that time, there were 45 deaths (10.1%). Sixteen patients died because of cardiac events (heart failure, myocardial infarction), seven patients died due to infectious disease (sepsis, pneumonia), five patients died from cancer, one patient died due to a major stroke, and for 16 patients the cause of death remained unknown. No death was procedure-related, four deaths occurred within the same hospital stay, and all other deaths occurred after hospital discharge. Kaplan-Meier survival estimates for all patients were 94.9% (±1.1) after 12 months and 87.8% (±2.0) after 24 months. Critical limb ischaemia (CLI) patients (RBC ≥4) had a significantly worse prognosis than patients with claudication: survival rates after one and two years were 97.4% (±0.9) and 90.9% (±1.9) for patients with claudication and 82.6% (±4.6) and 70.3% (±7.0) for CLI patients (p<0.001; Figure 2A). During the follow-up period, five major amputations (0.9%) and eight minor amputations (1.5%) occurred. Freedom from amputation after two years was 98.4% (±0.8) and 74.5% (±7.7) in claudicants and CLI patients, respectively (p<0.001; Figure 2B).
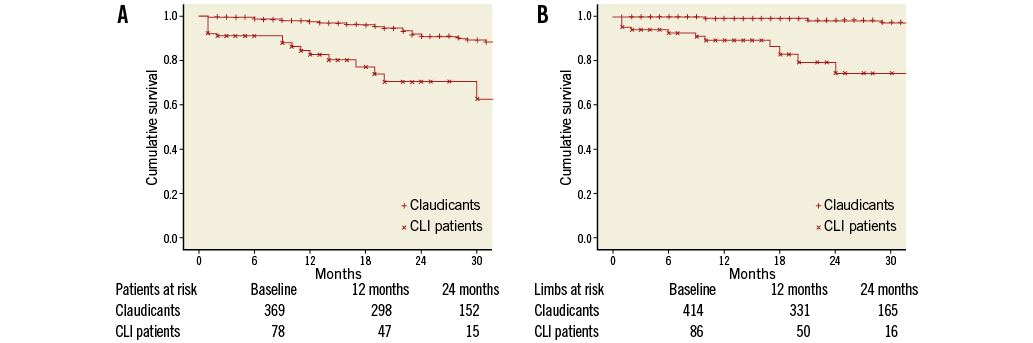
Figure 2. A) Survival rates for patients with claudication and patients with critical limb ischaemia (CLI patients). B) Freedom from amputation for patients with claudication and patients with critical limb ischaemia (CLI patients).
PERFORMANCE ANALYSIS
The primary endpoint of this study was binary restenosis (≥50%) as measured by the in-stent binary restenosis using duplex ultrasound (DUS) following treatment, six and 12-month follow-up visits. Following treatment, binary restenosis was not detected in any patient. There were no target vessel occlusions. At the six-month follow-up, duplex ultrasound showed that in 11 patients (11.8%) binary restenosis was present. There were no target vessel occlusions. At 12 months, six patients (7.9%) presented with binary restenosis by duplex ultrasound. There were two target vessel occlusions (2.2%), adding up to a total of eight patients with restenosis or reocclusion after 12 months (8.9%). However, in five of the eight patients restenosis was already detected at the six-month follow-up. Thus, there were only three new cases with restenosis or reocclusion at the 12-month examination.
The Kaplan-Meier estimates of primary, assisted primary and secondary stent patency at 12 months were 83.3%, 98.1% and 88.8%, respectively (Table 5). At two years, primary stent patency, secondary stent patency and freedom from TLR were 72.8%, 92.0% and 79.0%, respectively. The graphic displays of cumulative rates of primary and secondary stent patency and freedom from TLR are shown in Figure 3A. The influence of patient or lesion characteristics and procedural details on primary stent patency was analysed using Cox regression analysis. Patients with in-stent stenosis (p=0.04; 12-month PP 67.3%), impaired below-the-knee (BTK) run-off via collaterals only (p=0.04; 12-month PP 66.7%), and retrograde recanalisation (p=0.03; 12-month PP 66.3%) had a significantly higher risk of restenosis. There was a tendency towards more favourable patency rates in shorter lesions (Table 5), although this did not reach significance (p=0.06). PP rates of the SUPERA stent did not differ between the popliteal segments (p=0.35) or between SFA and popliteal lesions (p=0.88; Figure 3B).
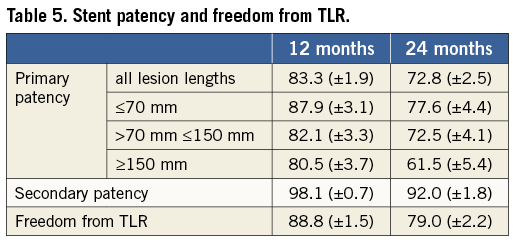
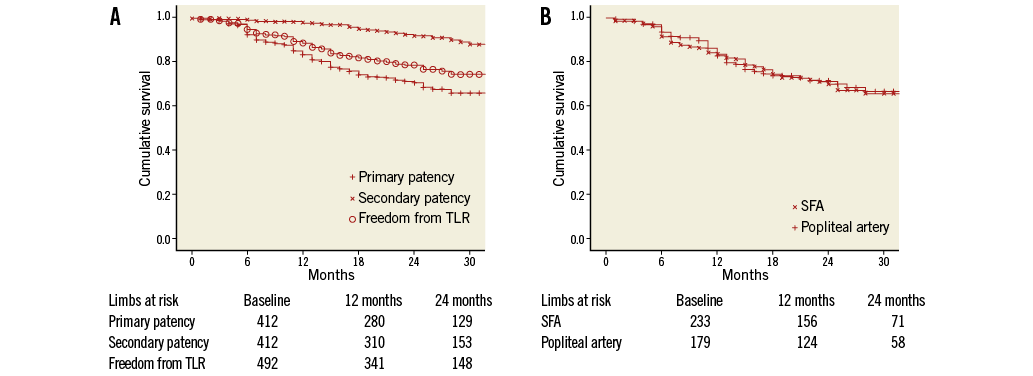
Figure 3. A) Cumulative rates of primary patency, secondary stent patency and freedom from target lesion revascularisation (TLR). B) Cumulative rates of primary patency for SFA lesions and popliteal lesions.
RUTHERFORD-BECKER CLASS, ANKLE-BRACHIAL INDEX AND X-RAY EXAMINATIONS
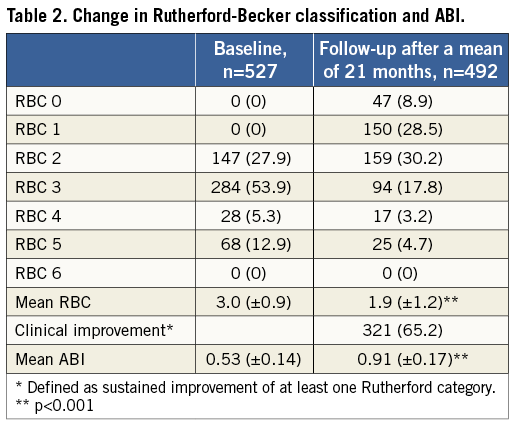
The mean RBC decreased from 3.0±0.9 at baseline to 1.9±1.2 after a mean follow-up of 21 months. Sustained improvement in at least one Rutherford classification was documented in 321 limbs (65.2%) (Table 2).
The ABI increased from 0.53±0.14 before the stent implantation procedure to 0.91±0.17 at 21 months (Table 3). These changes in RBC and ABI from baseline were highly statistically significant (p<0.001). Follow-up radiographs of 304 stents, obtained in 229 patients at a mean follow-up of 16.6 months, confirmed the absence of stent fractures in 100% of examinations.
Discussion
We report on the midterm results of a prospective cohort of patients treated with SUPERA vascular mimetic stents for symptomatic femoropopliteal artery disease. The PP rates of 83.3% and 72.8% after one and two years of follow-up, respectively, are very encouraging and are favourable compared to those reported in other SFA stenting trials3-5,12-15.
Maintaining patency after recanalisation of complex femoropopliteal lesions remains one of the greatest challenges of endovascular therapy. The introduction of nitinol stents for treatment of the SFA has resulted in markedly improved acute and midterm results at least for lesions with a length up to around 15 cm3,5,13,15. For longer lesions patency results with standard nitinol tubular stents were less convincing, and the reported issue of stent fractures with these nitinol stents raised concerns about the suitability of stenting for these complex lesions7,14.
Clearly these observations have also contributed to a widespread reluctance to implant stents into the popliteal artery. In fact, patients with isolated popliteal disease are largely underrepresented in the available literature. In a recent one-year analysis of the SUPERA stent in the popliteal artery16, the patency rates after one year were almost identical to the results obtained with the SUPERA stent in the SFA6 at a similar time point. In the current study, these results were corroborated in a much larger cohort of more than 500 limbs for a follow-up of two years. Moreover, also in the popliteal artery a complete absence of stent fracture has been demonstrated, providing reassurance that this novel stent design is suited similarly for implantation into the popliteal artery as well as the SFA.
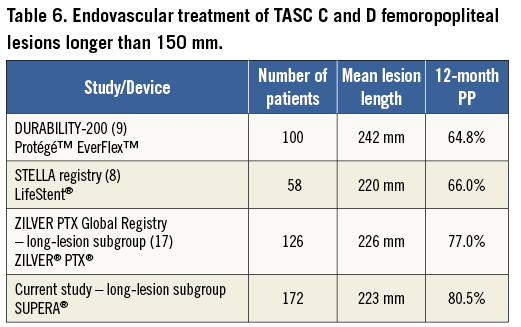
Endovascular treatment of long femoropopliteal lesions remains a controversial issue, and results in TASC C and D femoropopliteal lesions are scarce (Table 6). The DURABILITY-200 study9 demonstrated a 12-month PP rate of 64.8% with a 6% fracture rate, and in the STELLA registry8 the 12-month PP rate was 66.0% with a 17.7% fracture rate. A subgroup analysis17 from the Zilver® PTX™ Global Registry of lesions longer than 150 mm reported a 12-month PP rate of 77.0%. Compared to these data, the SUPERA stent performed very well with a 12-month PP rate of 80.5% in the long-lesion subgroup with zero fractures, confirming the durability of this device in extensive femoropopliteal disease.
Besides lesion length and location in the distal femoropopliteal segment, several other factors added to the complexity of the lesions treated in this patient cohort: more than 50% of lesions were total occlusions, which are known to be associated with markedly higher restenosis rates18. This is a higher proportion of total occlusions than in other contemporary SFA stenting trials3-5,13,15,19. Despite the high prevalence of severe or moderate arterial calcification (52.4%), acute luminal gain was achieved in 99.1% of the cases, which is attributable to the high radial strength of the SUPERA stent.
In a previous analysis6 of its use in the femoropopliteal segment, the SUPERA stent has been shown to have very good patency rates up to two years. However, this analysis was confined to 107 patients. The present study was performed on a larger patient population of more than 500 limbs and thereby allows identification of variables that might influence the device’s patency. Shorter lesions showed somewhat better results in terms of stent patency, although this effect did not reach significance. The following factors were identified as significant predictors of patency loss: treatment of an in-stent restenosis, retrograde recanalisation and impaired infrapopliteal run-off. While outflow via the infrapopliteal arteries is a known factor influencing the outcome of femoropopliteal procedures20, little is known on the outcome of retrograde techniques and about the best treatment strategy for in-stent stenosis21. The need for retrograde recanalisation seems to be a marker of more complex lesion morphology. The lower patency rate for patients following retrograde recanalisation confirms results from former studies about retrograde transpopliteal recanalisation22. These conditions underline the broad all-comers intention of this study, which included patients presenting with a wide variety of complex lesions in the femoropopliteal arteries.
Treatment of femoropopliteal arterial disease using a self-expanding interwoven nitinol stent resulted in a favourable midterm patency rate and durability. The SUPERA stent proved to perform well in a large variety of patients and lesions, especially in long lesions, and in patients with tissue loss and with a large proportion of calcified arteries and total occlusions, with no stent fractures observed. Sustained clinical improvement and a low TLR rate underline the efficacy of this device for the treatment of symptomatic femoropopliteal artery disease and also for CLI patients. Limitations of the data presented include the retrospective single-arm, single-centre design of the analysis. However, the large number of patients and the broad clinical spectrum of included cases without pre-specified inclusion and exclusion criteria give a good “real-world” impression of the performance of the SUPERA stent in the femoropopliteal segment and is one of the largest reported cohorts to date on stent-based treatment in this vascular bed. Further evidence, in the form of randomised controlled trials, is needed to confirm these encouraging results.
| Impact on daily practice Treatment of complex femoropopliteal lesions by stent implantation is limited by acute technical failure due to recoil and restenosis due to intimal hyperplasia. The SUPERA stent is designed to withstand the mechanical forces in the femoropopliteal arteries and – in the current study – proved to be efficient even in long and calcified obstructive lesions including the distal superficial femoral and popliteal artery. It is fracture resistant which makes it an ideal device, if stent treatment in the distal femoropopliteal segment is necessary. Future innovations in mechanical stent characteristics will possibly improve outcomes for this challenging arterial bed. |
Conflict of interest statement
D. Scheinert and A. Schmidt are consultants for IDEV Technologies. The other authors have no conflicts of interest to declare.

