Abstract
Aims: To evaluate a newly developed self-expanding Misago™ (Terumo Corp., Tokyo, Japan) nitinol stent with rapid exchange delivery catheter for the treatment of stenotic or occluded superficial femoral (SF) or popliteal arteries.
Methods and results: MISAGO 1 clinical trial enrolled 55 patients undergoing percutaneous intervention of totally occluded or stenotic lesions in SF or popliteal arteries treated with the implantation of 81 stents in five centres across Europe. Primary endpoint was restenosis rate at six months assessed by duplex sonography.
Patients (67% male) were 68±9 years old, 60% were smokers and 31% had diabetes. Clinical symptoms of ischaemia were present in all patients. Average lesion length was 85±50 mm, 64% were totally occluded and 38% classified as TASC C or D. The technical success rate was 100% while the procedural success rate was 98.2% without death, MI, stroke, or major bleeding. At six months follow-up the restenosis rate was 8.5%. One patient (1.8%) died of bronchial carcinoma and two (3.6%) underwent target vessel revascularisation. Mean ankle brachial index improved from 0.70 at baseline to 0.95 at six months while walking capacity on treadmill test improved with an average of 147 m. Rutherford index at six months demonstrated improvement of 72%, without any patients having symptom deterioration. One case of stent fracture was observed.
Conclusions: The results from this first-in-man study indicate good safety and short to medium term efficacy profile of the Misago nitinol stent.
Introduction
Balloon dilatation is still the most frequent non-surgical treatment of atherosclerotic lesions in the femoro-popliteal region. However, when used in more difficult lesions (long calcified stenoses, eccentric lesions), balloon angioplasty does not show satisfactory results at longer term follow-up1,2. Therefore, there was a clear need for further investigation and a search for new devices. Stent development was the most obvious response to the limitations of balloon angioplasty. Unfortunately, due to various reasons, long-term performance of stents in the superficial femoral artery (SFA) and popliteal artery (PA) appears to be more problematic than in other peripheral vascular beds, such as the iliac and carotid arteries. This may be caused by significant mechanical stresses placed on the devices, the vessel length and/or the severity of stenotic and occlusive disease3.
In-stent restenosis or stent patency and maybe stent fracture are important issues of SFA-PA stenting4. According to some recent publications, fracture rate seems to be reduced by improved flexibility of the new generation stents, the mechanical properties of the nitinol alloy and the specific stent design4,5. However in-stent restenosis still remains a problem.
This study describes the first clinical experiences with the Misago nitinol stent system, the first self-expanding stent with rapid exchange type delivery catheter in femoro-popliteal area.
Patients, materials and methods
From September 2006 until July 2007, a total of 55 patients were enrolled in the MISAGO 1 trial. Patients characteristics are summarised in Table 1.
All patients were treated with Misago peripheral stents in five hospitals located in France, Germany and Italy. The main inclusion criteria were: adult patients with symptomatic leg ischaemia (Rutherford scale 2+) due to stenosis or occlusion of the superficial femoral or popliteal arteries with reference diameter between 4 and 7 mm; de novo lesions either occluded or with at least 70% stenosis, including recoiling/ dissection/restenosis after balloon angioplasty; lesion length between 2 and 15 cm; at least one patent (less than 50% stenosis) run-off vessel.
Exclusion criteria were: previous bypass surgery or stenting in the target vessel; scheduled staged procedure of multiple lesions within the ipsilateral iliac or popliteal arteries within 30 days after the index procedure; co-existing aneurismal disease of the abdominal aorta, iliac or popliteal arteries; acute thrombophlebitis or deep venous thrombosis.
The study protocol was approved by the ethics committees of the participating hospitals and written informed consent was obtained from all patients before inclusion into the study.
Stent type
The Misago™ (Terumo Corp., Tokyo, Japan) is the first peripheral nitinol self-expanding stent system with rapid exchange (RX) type delivery catheter (Figure 1, 2, 3). The stent is made of a nickel-titanium alloy with three radiopaque markers at each end (for a total of 6). Misago stent has unique zig-zag cell design that in the preclinical tests showed good conformability and resistance to fracture.
For the study, stents of 40 and 80 mm in length with 6 and 8 mm diameter, were available.
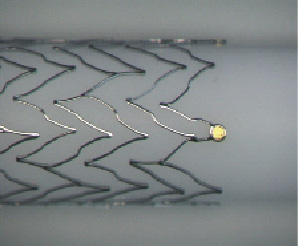
Figure 1. Expanded Misago stent - lateral view.
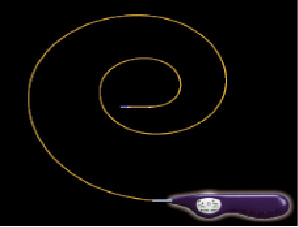
Figure 2. Misago stent system with RX type delivery catheter.
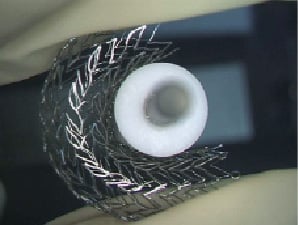
Figure 3. Preservation of inner stent diameter.
Procedure
The stenting procedure followed routine hospital practice and the Misago peripheral self-expanding stent system instructions for use. The investigators were encouraged to enrol all consecutive patients who fulfil inclusion criteria.
A maximum of one lesion, and two Misago stents per lesion were allowed. The lesion had to be covered from healthy to healthy segment.
Optimal expansion of the stent required it to be fully unfolded against the arterial wall with post implant residual stenosis less than 30%. The reference vessel diameter of the treated segment had to be 1 mm smaller than the implanted stent. Post-dilatation was recommended in cases where the stent did not expand to the proper diameter.
All investigators were requested to assess the stent based on the six predefined characteristics (ease of delivery, precise deployment, expanding force, vessel coverage, conformability, radiopacity) in the grading scale from one to five (one stood for worst and five for the best).
Protocol defined examinations included:
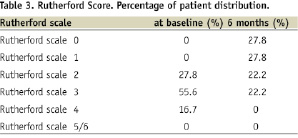
– Clinical evaluation: grading of peripheral arterial disease according to the Rutherford classification before procedure and at six months follow-up.
– Invasive imaging evaluation: angiography before and after stent placement on the same projection.
– Haemodynamic non-invasive evaluation: Ankle-Brachial Index (ABI) at rest and during treadmill exercise testing (3.2 km/hour at a 12% slope) before and after procedure and at six months follow-up.
– Non-invasive imaging test (colour-coded duplex sonography) at six months follow-up to determine restenosis.
The degree of restenosis by duplex sonography in the femoro-popliteal segment was classified using the peak velocity ratio. Peak velocity ratio was calculated by dividing the peak systolic velocity in the stenotic segment by the peak systolic velocity in the preceding normal segment or, in patients with multiple stenoses, in the adjacent distal segment. A peak velocity ratio of 2.4 was predefined as 50% stenosis.
Medication
Investigators were advised to give Clopidogrel 75 mg/day at least three days prior to the procedure, or alternatively 300 mg immediately after the procedure to all enrolled patients. Post-procedure, dual antiplatelet therapy with Clopidogrel (1 x 75 mg/day), or Ticlopidine (2 x 250 mg/day), was recommended for four weeks and 100 mg/day aspirin indefinitely.
Study endpoints
The primary study endpoint was the restenosis rate at six months (defined as more than 50% diameter stenosis) of the treated segment determined by duplex sonography.
Secondary endpoints included technical success defined as residual diameter stenosis of less than 30% post stent implantation; acute procedure success defined as event free technically successful procedure; acute complication rate defined as in-hospital death, stroke, bleeding requiring more than two units transfusion, or any other complication which was device or procedure related; ABI improvement (post-procedure versus pre-procedure and at six months versus before and after procedure); clinically driven target vessel revascularisation at six months; major complications at six months, including amputation of the distal part of the foot, the leg below the knee or the thigh; vascular and bleeding complications and stent fracture at six months.
Statistical analysis
MISAGO 1 is an observational study to validate the performance of the device. The statistical analysis was based on descriptive statistical techniques. Categorical variables are presented as rates whereas continuous variables are presented as mean ±1 standard deviation. All analyses were carried out by means of SAS software, version 9.3 (SAS Institute Inc, Cary, NC, USA).
Results
Procedural results
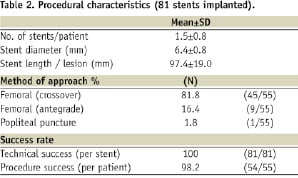
Acute procedure success was 98.2% (54/55) with one procedural complication during treatment of a long (150 mm), totally occluded (TASC D) lesion requiring implantation of two Misago stents (6 x 80 mm). To treat distal dissection, an additional stent was implanted. This complex procedure resulted in a thrombosis that was successfully resolved by thrombolytic therapy and additional stent implantation. Further details are shown in Table 2.
Between procedure and hospital discharge, one patient underwent clinically driven target vessel revascularisation due to a thrombotic occlusion between two previously implanted Misago stents (not overlapped) in the long lesion of the SFA-popliteal segment. After thrombolytic therapy and implantation of an additional stent, the angiogram revealed a completely patent vessel in the treated segment.
The treated vessels of both patients with in-hospital complications were fully patent at six months follow-up.
Immediate outcome
After stent implantation, the symptoms of ischaemia improved in all patients. The mean ABI of 0.70±0.21 before procedure increased with an average of 0.24±0.12 (p<0.001) after the stent implantation (Figure 4).
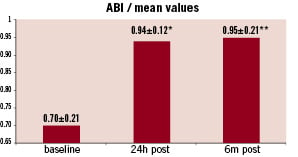
Figure 4. After stent implantation mean improvement of ABI at six months remained at a similar level as after procedure. * p<0.001 (for difference in means between baseline and 24 hours post-procedure) **p=0.001 (for difference in means between baseline and six months post-procedure)
Six months follow-up
Six months (±2 weeks) after the index procedure, systematic re-examination could be performed in 47 out of 55 patients (one patient died, three refused and four were lost to follow-up).
Clinical outcomes
At six months follow-up, 89 % of the patients were asymptomatic.
In 72.2% of the patients an improvement was demonstrated on the Rutherford scale and no patient showed a deterioration of the clinical symptoms (Table 3).
Haemodynamic non-invasive evaluation
Improvement of the ABI at six months was seen in 92.4% of patients. Only one patient had the same ABI value as at the baseline, and one patient showed deterioration on the ABI test. The latter patient had restenosis at six months follow-up.
Mean improvement of ABI at six months remained at a similar level as after the procedure (Figure 4). At six months, improved walking capacity was observed in 87.5% of the assessed patients. Mean increase in maximum walking capacity on treadmill test was 147±225 m (p=0.086) versus baseline.
Non-invasive imaging tests: duplex sonography
At six months follow-up, 47 patients were available for duplex ultrasound examination. In total 59 stents were analysed. In four stents a diameter stenosis of > 50% was documented. All other stents were fully patent. The primary endpoint, restenosis at six months, defined as loss of more than 50% of the functional luminal area inside the stent, was observed in 8.5% (4/47) of the patients. There was one (1.7%), type 1, stent fracture.
Adverse events
Two (3.6%) serious adverse events occurred between hospital discharge and six months follow-up. One patient (1.8%) died of bronchial carcinoma and one patient (1.8%) underwent angiographically driven, endovascular target vessel revascularisation.
No other major complications, including amputation of the distal part of the foot, the leg below the knee and the thigh, were reported in the study.
Discussion
In this first clinical experience, the Misago self-expanding peripheral stent demonstrated excellent technical and procedural success (100% and 98.2% respectively) combined with a good short to medium-term clinical outcome. A restenosis rate of 8.5%, one target lesion revascularisation and one stent fracture are promising results.
Recently published randomised clinical trials demonstrated the superiority of stenting versus angioplasty alone in longer SFA lesions6-9. However, reported fracture rates were up to 12%6. The main objectives of the new generation self-expanding nitinol stents were to overcome problems of stent fracture and to increase patency rates compared to previous generation SFA stents and angioplasty.
In 59 Misago stents assessed with duplex sonography, only one stent fracture (1.7%), neither clinically nor haemodynamically significant, was reported at six months.
Although follow-up was relatively short, it could be speculated that the low fracture rate is achieved by the enhanced flexibility of the stent resulting from the mechanical characteristics of the nitinol alloy and the segmental zig-zag stent design.
The restenosis rate of 8.5% at six months observed in our study is in line with previously reported results with similar stent types (SMART™ -bare metal stent [Cordis, Johnson & Johnson, Warren, NJ, USA], Luminexx™ [C. R. Bard, Inc., Murray Hill, NJ, USA], Lifestent® [C. R. Bard, Inc., Murray Hill, NJ, USA], Absolute Pro™ [Abbott Vascular, Redwood City, CA, USA]).4,6-10 The length of lesions treated in our trial was comparable or greater to the one reported in those studies,6-9 with the high percentage of total occlusions. Total occlusions are known to be associated with markedly higher restenosis rates,4 and their high prevalence of 64% in our patients allowed us to challenge this new stent.
Overall the incidence of adverse events in our study shows a favourable safety profile of the Misago stent up to six months follow-up.
The MISAGO stent is the first self-expandable nitinol stent on a RX delivery system which can be used with a 0.035´´ wire. This delivery system allows use of both normal and shorter wires which simplifies stent deployment, especially when performed by a single operator. The MISAGO-RX-stent could be considered as an alternative to other self-expanding nitinol stents with respect to the easier handling, but its place in the endovascular therapy will only be determined by the long-term results of studies enrolling larger number of the patients.
This study has several limitations, the main ones being the relatively small number of patients, the lack of randomisation and follow-up only until six months. Furthermore, four patients were lost to follow-up and three patients refused visit to the hospital at six months after the procedure. However, our study represents the first clinical experiences with this new stent and has given important and encouraging information about its safety and performance. A large multi-centre study, which enrolled more than 700 patients to be followed-up to one year is currently ongoing.
Conclusions
The results of the MISAGO 1 clinical trial showed high procedural success and promising short to medium-term safety and efficacy profiles of this new stent.
References

