Abstract
Aims: The primary patency of superficial femoral artery (SFA) stents is evaluated by measuring PSVR. However, each trial uses a different definition of PSVR. We investigated the impact of changing PSVR thresholds on the patency rates of SFA recanalisation with self-expanding nitinol stents.
Methods and results: A single-centre retrospective study was conducted. Between 2003 and 2006, 76 consecutive patients (83 limbs) were treated using nitinol self-expanding stents for SFA disease. Primary patency was defined as categories 1 (PSVR <2.0), 2 (PSVR <2.4) and 3 (PSVR <2.85). The mean follow-up time was 51±27 months. For one, five, and seven years, Kaplan-Meier estimates for primary patency rates were 62.6%, 36.8%, and 27.6%, respectively, in category 1; 75.2%, 46.5%, and 37.1%, respectively, in 2; and 75.2%, 46.1%, and 46.1%, respectively, in 3. The primary patency between categories 1 and 3 (p=0.038) was significantly different. No difference was observed between categories 2 and 3 (p=0.786), and a trend for differences was observed between categories 1 and 2 (p=0.069).
Conclusions: PSVR definition may influence the reported long-term patency rate of a SFA stent. We should consider the definition of restenosis in each trial.
Introduction
Trials to evaluate the use of newer nitinol self-expanding stents in superficial femoral artery (SFA) disease are underway. In all trials, primary patency was examined using duplex ultrasound (DUS). However, the definition of primary patency was different in each trial. For example, in the Zilver PTX clinical trial, the definition of primary patency was a duplex peak systolic velocity ratio (PSVR) of <21 and, in the DURABILITY I trial, the definition was a PSVR of <2.52. At present, many trials are being conducted, and DUS is employed for follow-up analyses. However, a different definition of primary patency is used in each trial. We aimed in this study to evaluate the impact of changing PSVR thresholds on the patency rates of SFA recanalisation with self-expanding nitinol stents.
Patient population and methods
STUDY POPULATION
A single-centre retrospective study was conducted. Between 2003 and 2006, 76 consecutive patients (83 limbs) were treated using nitinol self-expanding stents (SMART; Cordis Corporation, Miami Lakes, FL, USA) for symptomatic SFA disease (Rutherford categories 2-5) that affected their quality of life in spite of exercise and optimised medications3. The patients gave consent to a follow-up DUS examination at Kishiwada Tokushukai Hospital. We excluded patients who underwent stenting for restenosis and those who presented with acute limb ischaemia. The study protocol was designed in accordance with the Declaration of Helsinki, and it was approved by the ethics committee of our hospital. Written informed consent was obtained from every patient.
PROCEDURE AND MEDICATION
Endovascular therapy (EVT) was performed percutaneously, mostly using a crossover technique with a 6 Fr sheath. Prior to the intervention, 5,000 units of unfractionated heparin were administered to maintain the activated clotting time at more than 250 seconds. A 0.014 inch or 0.035 inch guidewire was advanced and the lesion crossed. An optimally sized balloon was employed and dilated. The stent size was selected to be 1-2 mm larger than the diameter of the reference vessel. Aspirin (100 mg/day) and clopidogrel (75 mg) or ticlopidine (200 mg/day) were administered two days prior to the procedure and were continued for at least one month.
FOLLOW-UP AND OUTCOMES
Patients received DUS within 30 days of the intervention and at six-month intervals thereafter. Lesion patency was evaluated by DUS examination.
High-resolution duplex scanning was performed on an ultrasound machine (Aplio; Toshiba Corporation, Tokyo, Japan) with a 7.5 MHz linear transducer by six investigators with at least two years of experience in peripheral vascular duplex scanning. PSVR was calculated by dividing velocity measured at the point of maximum stenosis by velocity in the closest adjacent normal vessel segment. The angle of incidence of the Doppler beam to the flow was maintained at less than 60 degrees.
Primary patency was defined by three categories. Category 1 was defined as PSVR of <2.00, category 2 was defined as PSVR of <2.40 and category 3 was defined as PSVR of <2.851,4,5. The complete absence of a detected signal was graded as a complete occlusion. Primary patency was defined as no restenosis or repeat revascularisation in the treated vessel.
STATISTICAL ANALYSIS
Statistical analysis was performed using SPSS (SPSS Inc., Chicago, IL, USA). Data are reported as mean±SD. Time-dependent outcomes were analysed using the Kaplan-Meier method and compared using the log-rank test. A p-value <0.05 was considered statistically significant.
Results
BASELINE CLINICAL AND LESION CHARACTERISTICS
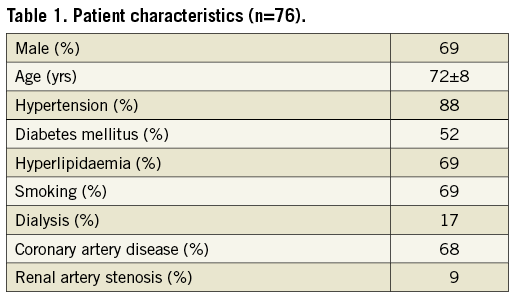
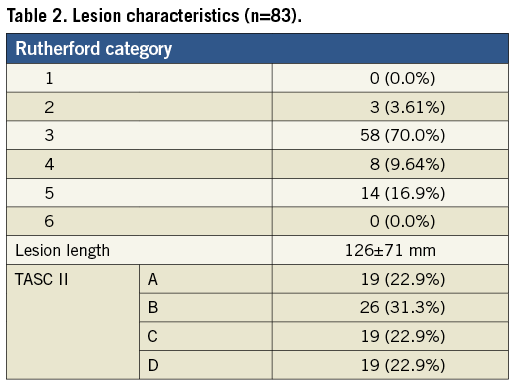
Between 2003 and 2006, 76 consecutive patients (83 limbs) were enrolled in this study. Patient characteristics are shown in Table 1. The mean age of the patients was 72±8 years; 69% of patients were male; 52% of patients had diabetes mellitus, and 17% of patients were on dialysis. Lesion characteristics are presented in Table 2. A total of 26.5% of lesions belonged to Rutherford categories 4 and 5. The average lesion length was 126±71 mm, and Trans-Atlantic Inter-Society Consensus (TASC) II A, B, C, and D were 22.9%, 31.3%, 22.9% and 22.9%, respectively.
OUTCOMES
The mean follow-up time was 51±27 months. In category 1 (PSVR<2.00), Kaplan-Meier analysis estimates for primary patency rates were 62.6%, 36.8%, and 27.6% for one, five, and seven years, respectively. In category 2 (PSVR<2.40), the estimates were 75.2%, 46.5%, and 37.1% for one, five, and seven years, respectively. In category 3 (PSVR<2.85), the estimates were 75.2%, 46.1%, and 46.1% for one, five, and seven years, respectively (Figure 1).
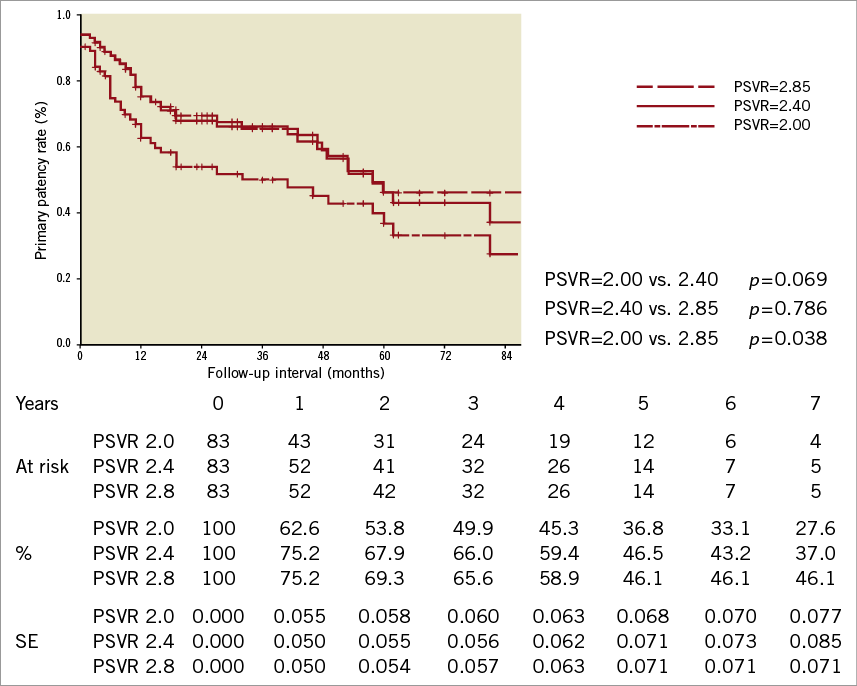
Figure 1. The primary patency between categories 1 and 3 (p=0.038, log-rank test) was significantly different. No difference was observed between categories 2 and 3 (p=0.786, log-rank test), and a trend for differences was observed between categories 1 and 2 (p=0.069, log-rank test). SE: standard error.
A significant difference in primary patency was observed between categories 1 and 3 (p=0.038, log-rank test). No difference was observed between categories 2 and 3 (p=0.786, log-rank test), and a trend for differences was observed between categories 1 and 2 (p=0.069, log-rank test).
Discussion
The use of EVT for lower extremity ischaemia has become widespread because of technological advances, and the strategy of revascularisation has shifted from surgical treatment towards EVT6-9. Considerable advances in EVT technology for SFA disease include the dramatic increase in the use of nitinol stents, which have become the standard of care to date because of the results obtained from randomised trials10,11.
Recently, many trials have been conducted on SFA stents, and some of these trials have been completed. We were able to select new-generation stents based on the results of these trials. When interpreting the results of recent SFA studies, we must consider the PSVR definitions used by each study. To clarify this confusion regarding PSVR definitions, we have conducted this study. There are conflicting data regarding the benefits of femoral stenting. Many studies are currently in progress to examine the use of other self-expanding stent platforms for patients with intermittent claudication and rest pain. However, each of these trials uses their own PSVR definition for the evaluation of the patency rate. In this report we show the impact of changing PSVR thresholds on the patency rates of SFA.
We have to do a rethink on the optimal PSVR to detect restenosis, but the problem with evaluating restenosis is that its definition is different in each trial. This could be the basis of confusing results in terms of the patency rate for each stent. However, each stent design has a different stiffness, which may influence the PSVR.
Study limitations
This was a retrospective study. The number of patients followed in the chronic phase was relatively small. This study might lack statistical power to confirm the differences between groups. Further investigation of new-generation stents is needed in the near future.
Conclusion
When we compare trials for the purpose of selecting a new stent that has a better patency rate with less restenosis, we should consider the definition of restenosis in each of the trials that we are examining.
Conflict of interest statement
The authors have no conflicts of interest to declare.

