Abstract
Aims: To investigate the impact of coverage of the deep femoral artery (DFA) by stents during endovascular treatment of proximal superficial femoral artery (SFA) lesions involving its ostium on the patency of both femoral arteries.
Methods and results: We retrospectively analysed 171 limbs of 143 patients who had been treated with femoral bifurcation stenting. According to the stenting strategies, 101 limbs with DFA coverage (group 1) and 70 limbs without DFA coverage (group 2) were compared. SFA and DFA patency was assessed by intra-arterial or computed tomography angiography or duplex ultrasound. Baseline characteristics were similar between groups, except for stent diameter. Flow limitations in DFAs were observed in 13 limbs divided similarly between the groups (8.9% vs. 5.7%, p=0.320). During a median follow-up of 18 months, there was a trend towards higher SFA patency at one and three years in group 1 (83% and 63% vs. 75% and 50%, p=0.124). DFA patency at one and three years was not different between groups (88% and 77% vs. 83% and 71%, p=0.488).
Conclusions: Jailing of the DFA origin by SFA ostial stenting did not result in a significant increase in the acute or later loss of patency in either femoral artery.
Introduction
The purpose of the present study was to compare the outcomes of stenting strategies with versus without stent coverage of the SFA ostium and the DFA origin in patients with SFA lesions involving the femoral bifurcation.
Methods
STUDY SUBJECTS
Between April 2004 and April 2012, 469 SFA lesions were treated by balloon angioplasty with stent implantation, due to claudication or critical limb ischaemia (CLI). Among them, 171 limbs of 143 patients who were treated with stenting at the femoral bifurcation lesions were retrospectively evaluated. The limbs were divided into group 1 (101 limbs) treated by SFA ostial stenting with coverage of DFA origin and group 2 (70 limbs) treated by stenting without DFA coverage (Figure 1). The femoral bifurcation was defined as the segment with the carina, distal CFA (1 cm proximal to the carina) and the proximal SFA (1 cm distal to the carina), as previously reported9. Before the angioplasty procedure, all patients underwent physical evaluations, ankle-brachial index (ABI) assessments, and imaging tests, including computed tomography (CT), magnetic resonance angiography, or colour duplex ultrasound. The institutional review board at the Severance Hospital, Yonsei University Health System, approved this study and waived the requirements for informed consent for this retrospective analysis.
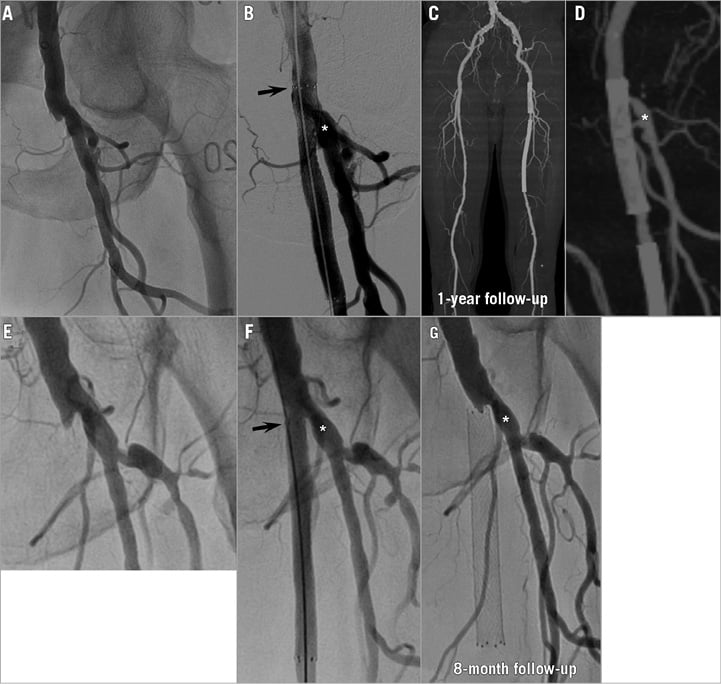
Figure 1. Representative cases from group 1 and group 2. Representative case from group 1. A 78-year-old man with total occlusion of the SFA ostium (A) underwent stenting with DFA coverage (B). Follow-up CT angiography at one year showed both femoral arteries patent (C & D). Representative case from group 2. A 70-year-old man with total occlusion of the SFA ostium (E) underwent stenting without DFA coverage (F). Angiography was performed at eight months because of recurring resting pain, showing total occlusion of the SFA with a patent DFA (G). The asterisk denotes the DFA, and the arrow denotes the proximal edge of the stent. CT: computed tomography; DFA: deep femoral artery; SFA: superficial femoral artery
ANGIOPLASTY PROCEDURE
All procedures were performed under local anaesthesia, which was supplemented with intravenous sedation and analgesia when required. Systemic heparin (5,000 IU) was administered to achieve an activated clotting time >250 seconds. For the treatment of occlusive SFA lesions, either the intraluminal or the subintimal approach was utilised at the physician’s discretion. Either ipsilateral or contralateral femoral puncture was performed, depending on the distance to the target lesion. A 7 Fr introducer sheath (Terumo Medical Corp., Tokyo, Japan) was used for the ipsilateral approach, and a 6-8 Fr contralateral sheath (Balkin; Cook Medical, Bloomington, IN, USA) was employed for the crossover approach. A 0.035-inch hydrophilic guidewire (Radifocus; Terumo Medical Corp.) and a supporting 5 Fr multipurpose catheter (Torcon NB®; Cook Medical) were used to cross the totally occluded lesion. For the subintimal approach, a subintimal channel was made with a straight 0.035-inch hydrophilic guidewire that was advanced distally, using a loop formed at the tip of the guidewire. The loop of the wire and catheter were advanced through the subintimal plane until the occlusion was passed and the wire re-entered the true lumen.
After the passage of the guidewire through the stenotic lesions, all of the target lesions were predilated with a balloon 5 or 6 mm in diameter. A self-expanding nitinol stent 5 to 8 mm in diameter (SMART; Cordis, Johnson & Johnson, Miami Lakes, FL, USA; Zilver®; Cook Medical; Complete® SE; Medtronic, Minneapolis, MN, USA; Absolute Pro®; Abbott Vascular, Santa Clara, CA, USA) was deployed. Limbs in group 1 were treated with stenting at the SFA ostium covering the origin of the DFA, and the limbs in group 2 were stented without covering the SFA ostium and DFA origin. Additional stents beyond the femoral bifurcation were selectively implanted in segments with residual stenosis >30% or dissections causing flow limitation. Deployed stents were dilated with the balloon for better apposition. Underexpanded stents were further dilated with a higher-pressure or larger-diameter balloon. The pressure gradient over the treated lesion was measured by the simultaneous recording of the mean systolic pressures of catheters which were positioned at the proximal and distal sides of the treated lesion. After successful recanalisation, all patients received a combination of aspirin (100 mg/d) and clopidogrel (75 mg/d), with or without cilostazol (200 mg/d), for at least one year.
Follow-up
For physical examinations, follow-ups were conducted at one month post procedure, and thereafter at three-month intervals. Non-invasive haemodynamic evaluations were repeated at six to 12-month intervals or if the symptoms deteriorated. At least one imaging test, such as CT angiography, colour duplex ultrasound, or intra-arterial angiography, was performed if a >0.15 decrement in the ABI was present on follow-up pulse volume recordings. Imaging tests were also employed if the symptoms worsened by one Rutherford category.
Study endpoints and definitions
The primary endpoints were the patency rates of SFAs and DFAs. The secondary endpoints included the cumulative target lesion revascularisation (TLR)-free survival and the cumulative major adverse events (MAE)-free survival rates. MAE was defined as the composite of all deaths and major amputations. Primary patency was defined as a treated vessel without restenosis and TLR. Significant binary restenosis was defined as ≥50% diameter stenosis based on intra-arterial angiography or CT angiography, or peak velocity >180 cm/s, or a lesion velocity to adjacent segment velocity ratio >2.4 by duplex ultrasound. For the patency of DFAs, only the limbs with follow-up intra-arterial angiography or CT angiography were analysed, because the duplex ultrasound follow-up did not routinely include DFA investigation. DFAs with a pre-existing significant stenosis prior to stenting were considered as patent if the stenosis did not worsen.
Statistical analysis
Continuous data are presented as mean±standard deviation, and categorical data are presented as frequency (%). Baseline clinical and lesion characteristics were compared between groups using the Student’s t-test for numerical data or Fisher’s exact test for categorical data. Primary and secondary endpoints were determined using Kaplan-Meier survival analysis and compared by log-rank test. The outcomes of both groups were censored at a fixed point of three years to avoid any bias that may have been caused by follow-ups of varying durations.
Subgroup analyses that were stratified by selected clinical and lesion characteristics were performed for the primary endpoint using a test for heterogeneity to assess for possible interactions between the stenting strategies and various characteristics. Findings were considered significant if the p-value was less than 0.05. All statistical analyses were performed with SPSS 18.0 (SPSS Inc., Chicago, IL, USA).
Results
BASELINE CHARACTERISTICS
The baseline characteristics of the treated limbs are summarised in Table 1. The mean age was 70±8 years, and 81.9% of the limbs belonged to male patients. Of the 171 treated limbs, 61 limbs (35.7%) had CLI. Baseline clinical characteristics were not different between the two groups (Table 1).
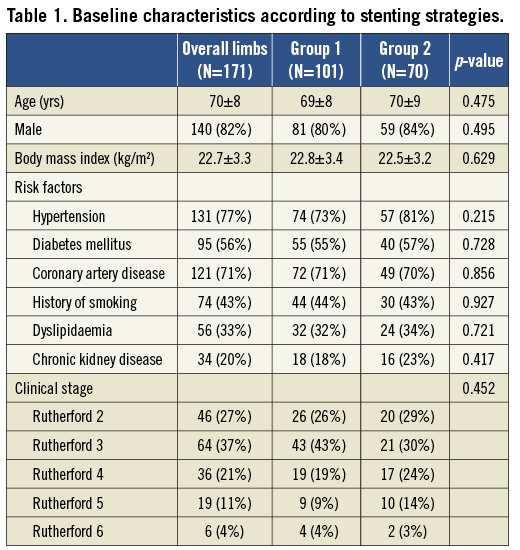
The mean lesion length was 22.5±8.4 cm, and it did not differ between the two groups (Table 2). In 49 SFA lesions (28.7%), additional stents were implanted beyond the femoral bifurcation. The limbs of group 1 tended to show a greater, but not statistically significant, involvement of CFA or DFA lesions. Most of the treated lesions were TASC II classification C or D (88.3%) and chronic total occlusion (CTO) lesions of the SFA ostium (77.8%). Pre-procedural ABI was lower in group 1 than in group 2 (0.45±0.18 vs. 0.51±0.17, p=0.046). Subintimal angioplasty was performed at similar frequencies in both groups (66.3% and 71.4%, p=0.481). Larger diameters of stents were implanted at the bifurcation in group 1 than in group 2 (7.5±0.7 vs. 7.1±0.7 mm, p<0.001). Other angiographic and procedural characteristics were similar between the two groups (Table 2).
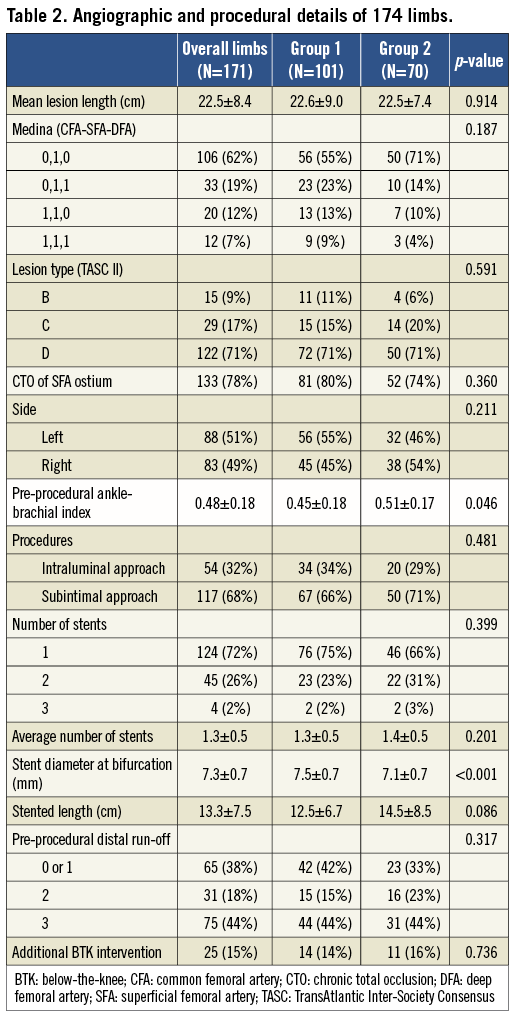
IMMEDIATE PROCEDURAL OUTCOMES
Post-procedural ABI was increased from 0.48±0.18 to 0.83±0.19 (p<0.001). There were no procedure-related deaths in either group. However, nine procedures (5.3%) were complicated by distal embolisation, which blocked the distal flow: four limbs were treated with surgical embolectomy, and five limbs were successfully treated with aspiration or the catheter-based administration of thrombolytics. Arterial perforation occurred in two (1.2%) limbs: one limb was managed conservatively, and the other was successfully treated with a covered stent. There were no significant differences in the frequency of complications between the two groups (Table 3).
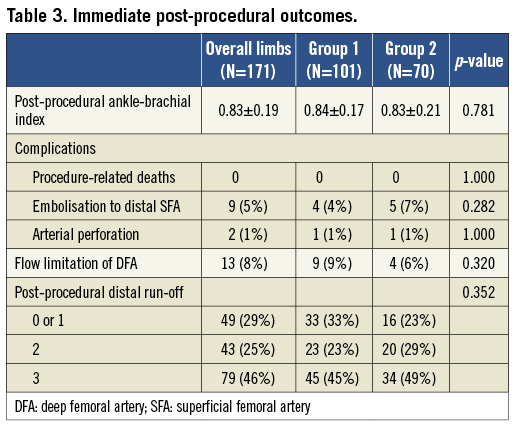
Flow limitations in DFAs after stenting were observed in 13 limbs (7.6%), nine limbs (8.9%) in group 1 and four limbs (5.7%) in group 2. There were no significant differences in flow limitations between the two groups (p=0.320, Table 3). However, there was a significant difference in the incidence of DFA flow limitation, according to the Medina classification (p<0.001, Figure 2). Post-procedural flow limitation was observed mainly in Medina type 0,1,1 (n=9, 27%) or 1,1,1 (n=2, 17%). Therefore, DFAs containing stenoses at their origin prior to the procedure appeared to be at a higher risk of flow limitation after the procedure. Of 13 limbs with flow-limiting jailed DFA, one DFA in group 1 showed restored blood flow after balloon angioplasty. The other 12 DFAs were not treated with additional interventions. Regarding the fate of the 12 DFAs with immediate flow limitations, five DFAs had follow-up imaging tests. Three DFAs remained occluded, but two DFAs regained patency during the follow-up.
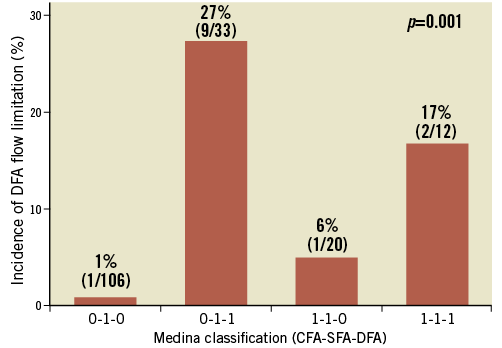
Figure 2. Incidence of DFA flow limitation after stenting according to the Medina classification. CFA: common femoral artery; DFA: deep femoral artery; SFA: superficial femoral artery
PATENCY RATES AND CLINICAL OUTCOMES, ACCORDING TO THE STENTING STRATEGIES
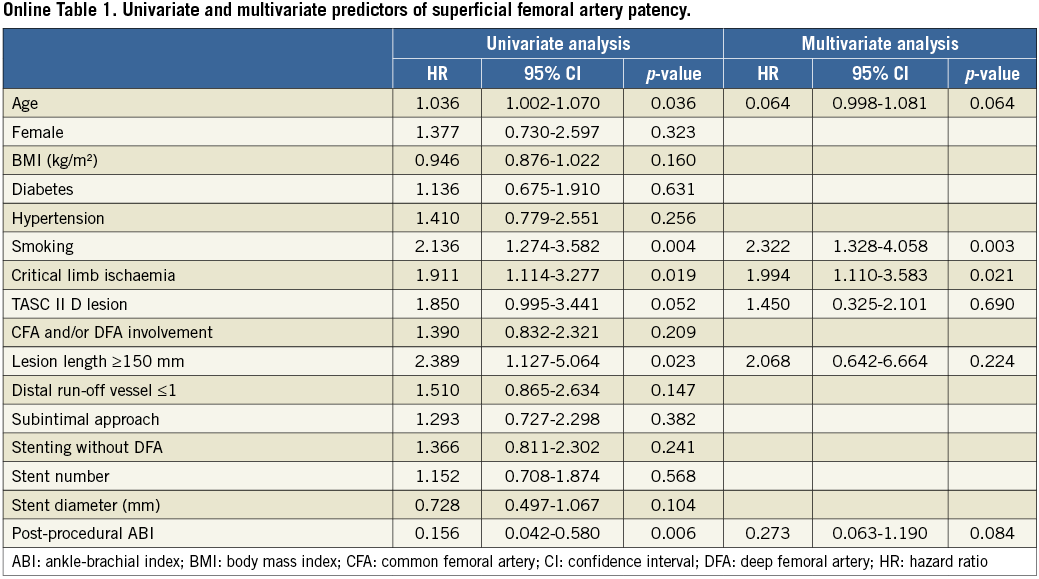
During the median follow-up period of 17.8 months (interquartile range, 6.0-30.5 months), binary restenosis at the target lesion was observed in 59 limbs, 34 limbs (33.7%) in group 1 and 25 limbs (35.7%) in group 2. Complete reocclusion was observed in 51 limbs, 28 limbs in group 1 and 23 limbs in group 2. Kaplan-Meier curves showed a higher tendency of SFA patency for group 1 compared to group 2 (p=0.124 by log-rank, Figure 3A). Patency rates of SFAs at one and three years were 83% and 63% for group 1 and 75% and 50% for group 2, respectively. Also, on multivariate analysis, the stenting strategy did not remain as an independent predictor for SFA patency (Online Table 1).
For the analysis of DFA patency, we excluded 57 limbs that did not have follow-up imaging tests performed for assessing the patency of proximal DFAs. Of 114 DFAs, 12 were immediately occluded after stenting without any interventions, and seven (two in group 1, five in group 2) demonstrated worsening stenosis during the follow-up. Patency rates of DFAs at one and three years were 88% and 77% for group 1 and 83% and 71% for group 2. There were no significant differences in the patency of DFAs between the two groups (p=0.488 by log-rank, Figure 3B). None of the worsened stenotic lesions of DFAs underwent revascularisation during the follow-ups.
Of the 59 limbs which developed restenosis or reocclusions of the SFA during the follow-up, 32 limbs underwent repeat percutaneous revascularisation, and five limbs underwent bypass surgery. The other 22 limbs were treated medically due to the refusal of the patient to undergo repeat revascularisation (n=5), poor medical condition of the patient (n=5), or mild symptoms (n=12). Percutaneous revascularisation was successful in 30 out of 32 limbs (93.8%). No limbs had any limitation for access for repeat percutaneous interventions or anastomosis site for bypass surgery. The revascularisation attempt failed in two limbs, because the wire could not pass through the totally occluded lesions. Group 1 showed a trend towards higher TLR-free survival rates at one and three years, compared to those of group 2 (88% and 78% vs. 80% and 64%, respectively, p=0.086 by log-rank, Figure 3C).
There were 13 mortalities during the follow-up. The causes of mortalities were cardiac (n=2), malignancies (n=2), infections (n=7), rupture of abdominal aortic aneurysm (n=1), and trauma (n=1). One limb required a planned below-the-knee amputation after a successful angioplasty. There were no significant differences in MAE between the two groups (p=0.151 by log-rank, Figure 3D).
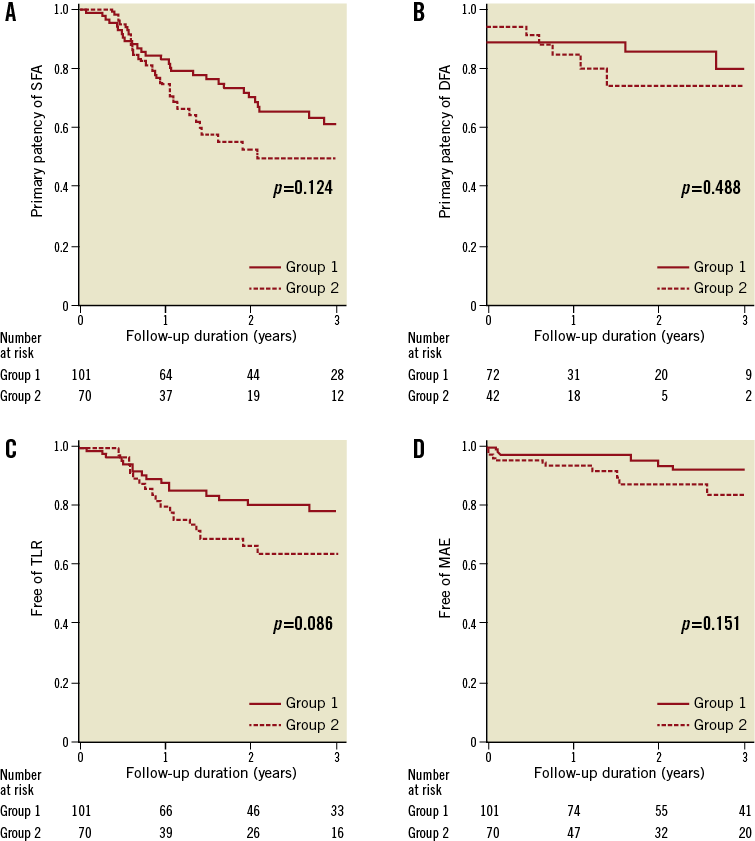
Figure 3. Kaplan-Meier survival curves of clinical endpoints according to the stenting strategies. The graphs depict primary patency of the SFA (A), primary patency of the DFA (B), TLR-free survival rates (C), MAE-free survival rates (D). DFA: deep femoral artery: MAE: major adverse events; SFA: superficial femoral artery; TLR: target lesion revascularisation
SUBGROUP ANALYSES FOR SFA PATENCY
Interactions between the stenting strategies and clinical parameters, including lesion characteristics, were estimated (Figure 4). The hazard ratios (HR) of SFA patency were not different between the two groups, according to the subgroups of age, sex, diabetes, hypertension, smoking, and the presence of CTO.
However, group 1 had higher patency rates than those of group 2 in the following subgroups: 1) lesion length less than 15 cm, 2) lesion involvement of CFA or DFA, and 3) intraluminal approach. Also, we observed a significant interaction between the stenting strategies and type of approach for predicting SFA patency (p=0.030 for interaction, Figure 4). For an intraluminal approach, the HR for group 2 versus group 1 was 3.92 (95% confidence interval [CI], 1.37-11.25, p=0.011). On the contrary, the HR was 1.03 (95% CI: 0.54-1.97, p=0.918) for the subintimal approach. In addition, we observed a significant interaction between the stenting strategies and lesion length. Stenting with DFA coverage showed higher SFA patency rates in subgroups of short lesions, compared to those in subgroups of long lesions (p=0.032 for interaction).
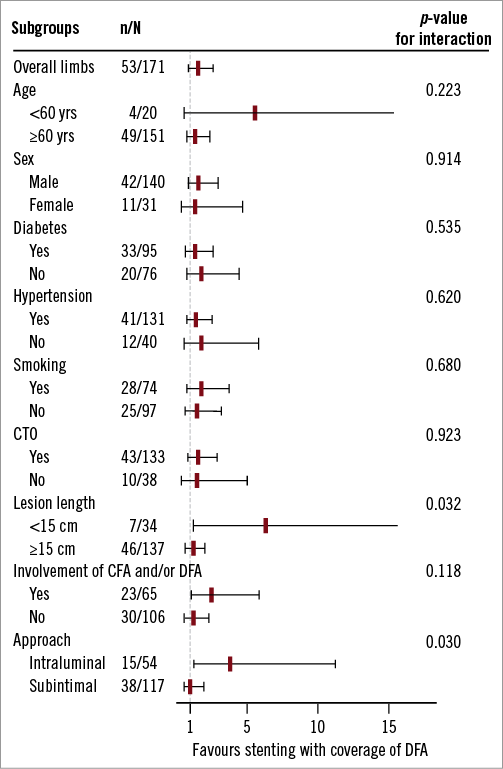
Figure 4. Forest plot of subgroup analyses of SFA patency comparing the stenting strategies. The test for interaction was significant within strata formed by lesion length and approach method. CFA: common femoral artery; CTO: chronic total occlusion; DFA: deep femoral artery
Discussion
The principal findings are: 1) in patients with SFA lesions involving the femoral bifurcation, ostial stenting at SFAs with coverage of the DFA origin showed a trend towards higher SFA patency and TLR-free survival rates, compared with the stent implantation strategy that lacked coverage of the SFA ostium and DFA origin, 2) jailing of the DFA origin by SFA ostial stenting did not significantly increase the acute or later loss of patency in DFAs, and 3) an improvement of the SFA patency by ostial stenting was associated with the subgroup treated with an intraluminal rather than a subintimal approach and the subgroup with short or intermediate rather than longer lesions.
STENTING AT THE FEMORAL BIFURCATION LEVELS AND THE EFFECT OF THE STENTING STRATEGIES ON THE PATENCY OF SFA
In the present study, stent implantation at the femoral bifurcation that covered the SFA ostium and DFA origin showed a trend towards higher patency and TLR-free survival rates, compared to stenting without coverage of the SFA ostium and DFA origin. In general practice the implantation of stents that proximally extend to the CFA is avoided because of the potential interference with blood flow to the DFA. Furthermore, the risk of stent fracture and the difficulty of further femoral vascular access have led to operator reluctance in stenting at the CFA. Thus, surgical treatment has been considered as the preferred treatment option for CFA disease5-8. However, recent studies have demonstrated the feasibility and favourable outcomes with endovascular treatment of CFAs, although these studies involved a relatively small number of procedures10,11. Moreover, Bonvini et al showed a higher patency rate after stenting at the CFA, compared to that of balloon angioplasty alone12.
Similar to our findings, Yamawaki et al recently reported a significantly higher patency of SFA using stenting with DFA coverage, compared to without DFA coverage, at one year9. The inferior outcomes of SFA stenting without coverage of the SFA ostium and DFA origin may be explained by the presence of residual plaque at the CFA and SFA ostium uncovered by the proximal edge of the stent, weak radial forces on a residual plaque at the edge of the stent, and rheological abnormalities at the femoral bifurcations. In particular, balloon injury of the diseased ostia of the SFA and DFA without stent implantation may lead to increases in recoil and neointimal hyperplasia. In our study, the differences between the two stenting strategies did not reach statistical significance, possibly due to the inclusion of more complex lesions, such as higher CTO lesions and TASC II D lesions, which were excluded from the previous study9.
In our study, we evaluated the presence of stent fractures in only 56 limbs with follow-up fluoroscopic images, and 10 stent fractures (18%) were identified. The incidence of stent fracture was not higher in group 1 compared to group 2 (7/48, 14.6% for group 1 vs. 3/8, 37.5% for group 2, p=0.143). Also, most stent fractures were type I with single tine fracture (n=7, 70%). There were three fractures, one type II and two type III.
THE FATE OF DFAs ACCORDING TO THE STENTING STRATEGIES
Another major concern regarding the implantation of stents at the femoral bifurcation is the jailing of the side branch, DFA, which is the most important collateral vessel for bypassing the obstructed SFA and is essential for maintaining limb viability13,14. In our study, flow limitations in post-procedural DFAs were observed in only 13 out of 171 limbs (7.6%). There were no significant differences between the two groups, and DFAs with pre-procedural stenoses at their origin appeared to be at a higher risk of flow limitation after the procedure. Also, the differences in stenting strategies did not significantly affect DFA patency during the follow-ups. Both groups had an acceptable, high DFA patency (88% at one year and 77% at three years in group 1, and 83% at one year and 71% at three years in group 2). Thus, coverage of the DFA by the stent did not appear to be associated with an increased risk of DFA occlusion, as long as flow limitations in the DFA did not occur. Additionally, limbs with impaired blood flow in the DFA did not develop any significant symptoms immediately after the procedure or during later follow-ups.
STENTING STRATEGIES ACCORDING TO THE LESION CHARACTERISTICS
Interestingly, there were interactions with lesion length and involvement of CFA and/or DFA with borderline significance. This suggests that stenting to the distal CFA with DFA coverage may be necessary.
In subgroups using the subintimal approach, there were no significant differences in the patency of SFA, according to the stenting strategies. However, in subgroups using the intraluminal approach, stenting with DFA coverage showed higher patency. These findings could be attributed to differences in plaque shift at the femoral bifurcation, depending on the type of approach. With the subintimal approach, the plaque-free subintimal channel becomes eccentrically expanded during balloon dilation while the true lumen gets compressed. However, with the intraluminal approach, the circular plaque burden at the SFA ostium may remain more or less at the same site while the whole vessel expands outward during balloon angioplasty. Therefore, there will be more remaining circular plaque burden in cases of intraluminal balloon angioplasty. In addition, vessel recoil force may be greater after intraluminal angioplasty than after subintimal angioplasty. For these reasons, stenting at the SFA ostium may be more important for the SFA patency after intraluminal angioplasty than after subintimal angioplasty.
Study limitations
There are several limitations to this study. First, only registry data from a single centre were analysed, comprising a relatively small group of patients with the inherent limitations associated with a retrospective design. Second, the imaging tests for assessment of DFA patency were not performed routinely. Most imaging tests were performed if there was a decline in ABI or a deterioration of symptoms. Therefore, the DFA patency rate may be inaccurate and overestimated. Third, detailed geometric analyses of potential plaque shift using intravascular ultrasound were not performed. Despite these limitations, we believe that our data represent the reality of current daily practice.
Conclusions
SFA ostial stenting with coverage of DFA origin was safe and showed relatively favourable patency rates in both femoral arteries. In subgroups with lesions of short/intermediate length (<15 cm) or treated with intraluminal angioplasty, SFA ostial stenting with DFA coverage showed higher SFA patency than stenting without DFA coverage.
| Impact on daily practice Implantation of stents at the femoral artery bifurcation covering the origin of the DFA has been generally avoided because of uncertainty about the fate of the jailed DFA. However, based on our study results, SFA ostial stenting with jailing of the DFA origin can be performed without concern when the femoral artery bifurcation is involved, because it is safe and shows a trend toward a higher patency than SFA stenting without covering the SFA ostium and the DFA origin. |
Funding
We gratefully acknowledge financial support for this study from: the Healthcare Technology R&D Project, Ministry for Health, Welfare & Family Affairs, Republic of Korea (No. A085012, A102064, and A110879); the Korea Health 21 R&D Project, Ministry of Health & Welfare, Republic of Korea (No. A085136); and the Cardiovascular Research Center, Seoul, Republic of Korea.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Online data supplement