
Abstract
Aims: Surgical endarterectomy is the therapy of choice for atherosclerotic common femoral artery (CFA) obstruction. Recently, some large single-centre series have shown encouraging results for the percutaneous treatment of CFA obstructions. The purpose of this study was to evaluate the safety, feasibility, and one-year efficacy of the endovascular treatment of CFA obstructions with combined use of directional atherectomy (DA) and a paclitaxel-coated balloon (DCB).
Methods and results: Between January 2012 and July 2014, 30 consecutive patients with severely calcified obstructions of the common femoral artery were treated in our centre using DA followed by DCB dilatation. Provisional stenting was allowed in the case of a suboptimal result. Twenty cases (66%) were isolated CFA interventions, whereas five (17%) and five (17%) also involved inflow and outflow vessels, respectively. Chronic total CFA occlusions (CTO) were recanalised in six cases (20%). Procedural success was achieved in all cases; stenting was needed in three cases (10%). At one year, restenosis and target lesion revascularisation were observed in two of 30 (6.6%) and one of 30 (3.3%) patients, respectively. The secondary patency rate was 96.7%.
Conclusions: This single-centre prospective study suggests that the combined use of DA and DCB is a safe and effective alternative to surgery, a treatment option for common femoral artery lesions and provides encouraging results in this setting.
Introduction
Atherosclerotic common femoral artery (CFA) obstruction is a known cause of symptomatic peripheral arterial disease and is usually part of a broader atherosclerotic involvement including the aortoiliac or femoropopliteal territories1. Although percutaneous treatment has been accepted as the preferred initial revascularisation strategy for the majority of atherosclerotic obstructions in the lower limb, CFA disease remains a mainly surgical domain because it is easily accessible, and endarterectomy is associated with favourable long-term outcomes2. In addition, it has to be borne in mind that there are some caveats to endovascular therapy of the CFA (i.e., loss of femoral vascular access or unfavourable lesion characteristics). Moreover, in up to 40% of CFA lesions treated with the endovascular approach, a provisional stent placement over the joint space is necessary3.
In recent years, improvements in endovascular equipment and in the technical skills of operators have led to an increasing number of percutaneous CFA interventions. Endovascular therapy for the treatment of obstructive disease of the CFA is associated with a high rate of acute technical success3,4, and several different interventional strategies5, including the use of a bioabsorbable stent6, have been proposed to improve patency rates.
The presence of severe calcification of the atherosclerotic lesion is responsible for a poor response to balloon dilation, due to significant acute vessel recoil and frequent flow-limiting dissections.
Directional atherectomy (DA) improves acute success by debulking the fibrocalcific portion of the atherosclerotic plaque and could provide some benefits in terms of the patency rate2.
The combined use of DA and drug-coated balloons (DCB) for the endovascular treatment of calcified lesions of the femoropopliteal tract has been associated with good one-year patency7. At present, there are no data available regarding the combined use of DA and DCB in calcified lesions of the CFA.
The purpose of this study was to evaluate the technical feasibility, safety, acute and one-year efficacy of the endovascular treatment of atherosclerotic CFA obstructions with combined use of DA and DCB.
Methods
STUDY DESIGN
This was a single-centre registry. Between January 2012 and July 2014, 89 patients underwent revascularisation of the CFA in our institution due to critical limb ischaemia (CLI) or lifestyle-limiting claudication (LLC). Of these, 22 were treated surgically and 67 underwent percutaneous transluminal intervention (PTA) according to the TASC II recommendation. Within this cohort, 30 patients underwent PTA of a calcified lesion with the use of DA and DCB2,4.
The anatomic inclusion criteria were:
– Calcified lesion located at the CFA
– Diameter stenosis >70%
– Vessel diameter of 5 to 7 mm
The anatomic exclusion criteria were:
– Angiographic evidence of intraluminal thrombosis
– Spontaneous and/or iatrogenic dissection
– In-stent restenosis
– Length >5 cm
– Calcification grade <2
Calcifications were detected both with duplex scan and with fluoroscopy: grade 0=absence of any evidence of calcifications; grade 1=calcifications at one side of the lumen with length <1 cm; grade 2=calcifications at both sides of the lumen <1 cm; grade 3=calcifications at both sides of the lumen >1 cm in length7.
A total of 37 patients were excluded from analysis for the following reasons: complex aortic bifurcation anatomy precluding a safe crossover placement of the required sheath (7-8 Fr) (n=19), long ipsilateral SFA occlusion with surgical indication (n=5), in-stent restenosis (n=5), iatrogenic dissection/thrombosis of the CFA (n=4), and stent located at the ostium of the ipsilateral SFA.
CFA lesions were considered as bifurcation lesions and classified accordingly8.
Endovascular treatment included the combined use of directional atherectomy (TurboHawk™ Plaque Excision System; Covidien [now Medtronic, Minneapolis, MN, USA]) and a prolonged (3 minutes) paclitaxel-coated balloon dilation (IN.PACT™ Admiral™; Medtronic) in all cases.
CONCOMITANT THERAPY
All patients received aspirin (75-160 mg/day) and should have been on ticlopidine (250 mg twice daily) for at least seven days, or clopidogrel (75 mg/day) for at least four days. Alternatively, patients received a clopidogrel preload (300 mg) 24 hrs before the procedure.
Post procedure, thienopyridines were continued for 180 days, whereas aspirin was continued for life. For anticoagulation, 70-100 IU/kg of heparin was administered before wiring the lesion, with the intention of achieving an ACT of >250 s. Additional heparin was administered at the operator’s discretion according to ACT values9.
PTA TECHNIQUE
All procedures were performed percutaneously, with the patient under local anaesthesia. Vascular access was achieved via the contralateral common femoral artery. A 55 cm 8 Fr long sheath (Cook Medical, Bloomington, IN, USA) was used in order to achieve adequate support and to allow continued flushing with saline and/or contrast medium injection while using the TurboHawk.
Once diagnostic angiography was completed, a wire (0.014”), chosen by the operator according to the stenosis type, was navigated into the distal superficial femoral artery. In the case of total occlusion, a 0.018” or 0.35” wire was used to cross the lesion and was then replaced by a 0.014” wire. Balloon predilation was used only in the case of total occlusion (n=6) with an undersized balloon just to allow the filter and TurboHawk to get through the lesion. All recanalisations were performed using the intraluminal technique. In order to avoid embolisation of atherosclerotic debris, a filter for distal protection (SpiderFX™; Medtronic) was placed distal to the stenosis prior to the use of the peripheral DA.
After filter placement DA was performed. When the DA device nose was filled, it had to be removed and the atherosclerotic plaque removed from the storage nose cone. The number of cutting passages was decided solely by the operator. No part of the atherectomy system was blocked by calcified debris requiring the use of an additional device.
When the profunda or the superficial femoral artery was affected, the DA was performed in each of them.
When angiograms demonstrated that residual stenosis was lower than 30%, a post-dilation with a DCB (sizing was 1:1 to the reference vessel diameter and 10 mm longer than the stenosis) for at least 180 s was performed. The DCB device used was the IN.PACT Admiral PTA balloon10. This is a 0.035” peripheral balloon catheter coated with a matrix consisting of a drug (paclitaxel) combined with a hydrophilic spacer (urea). All these components work when the balloon contacts the vessel wall and the drug is freed, then pressed into the vessel wall; this allows an effective transfer controlled by how the drug is loaded onto the balloon and inhibits tissue growth within the artery, a factor that leads to the re-narrowing of arteries. Provisional stenting was allowed in the case of a suboptimal result after prolonged balloon dilatations, i.e., flow-limiting dissection, abrupt vessel occlusion or residual stenosis >50%.
POST-PROCEDURAL PATIENT MANAGEMENT
Femoral sheaths were removed when the ACT was <150 s. Access-site haemostasis was achieved by manual compression in all patients. If clinical signs of limb ischaemia occurred on the side of femoral access, sheaths were removed independently of post-procedural time and ACT values. Femoral sheath-induced leg ischaemia was classified as major if it required embolectomy, and minor if it was resolved by sheath removal. A complete blood count was obtained before the procedure and prior to hospital discharge9.
PATIENT FOLLOW-UP
Patients were evaluated up to hospital discharge, at 30 days, and at three, six and 12 months post procedure. The pre-interventional work-up and the follow-up visits at each interval included physical and clinical examination, assignment of a Rutherford classification, arterial Doppler occlusion pressure measurements with calculation of the ankle-brachial index (ABI), and colour duplex sonography measuring peak systolic velocities (PSV) for the calculation of the proximal peak systolic velocity ratio (PSVR)11.
DEFINITIONS
Procedural time was defined as the time from the completion of diagnostic angiography to the final views. Technical success was defined as the ability to pass the lesion with the guidewire and to perform DA and DCB post-dilation successfully with a residual stenosis <30%. Procedural success was defined as technical success without the occurrence of any major adverse events (MAE).
ENDPOINTS
The primary endpoint was freedom from target lesion revascularisation (TLR). Major adverse events (MAE) included: target lesion revascularisation (TLR), any death, target vessel revascularisation (TVR), myocardial infarction (MI), and unplanned index limb amputation at one year post procedure. Post-procedural angiography was performed when arterial duplex evaluation of the proximal flow velocity was between 2.5 and 5.0 (intermediate restenosis) and the patient had clinical symptoms, or when PSVR was greater than 5.0 (severe restenosis) regardless of the presence of clinical symptoms.
The secondary endpoints included: 1) primary patency at one year documented by duplex ultrasound (patency defined as a proximal PSVR <2.5), 2) clinical success as defined by >1 clinical category improvement in the Rutherford scale (or equivalent) from baseline (or two categories if there was pre-existing tissue loss) at one year, and 3) haemodynamic success, defined as a 0.1 improvement in the ABI during the period from baseline to 30 days post procedure and no deterioration >0.15 from the maximum early post-procedure level at one year.
STATISTICAL ANALYSIS
Nominal and categorical variables were presented as contingency tables with frequencies and percentages. Data were analysed using IBM SPSS Statistics for Windows, Version 23.0 (IBM Corp. Armonk, NY, USA). Continuous variables were reported as the mean with standard deviation. Variables not normally distributed were reported as medians and IQRs. The Shapiro-Wilk test was used to test the distribution of the variables for normality. Further testing was only carried out to analyse change over time in ABI and Rutherford classification. These variables were normally distributed and therefore a paired t-test was used (a probability value <0.05 was considered statistically significant).
Results
Patient and lesion characteristics are summarised in Table 1 and Table 2.
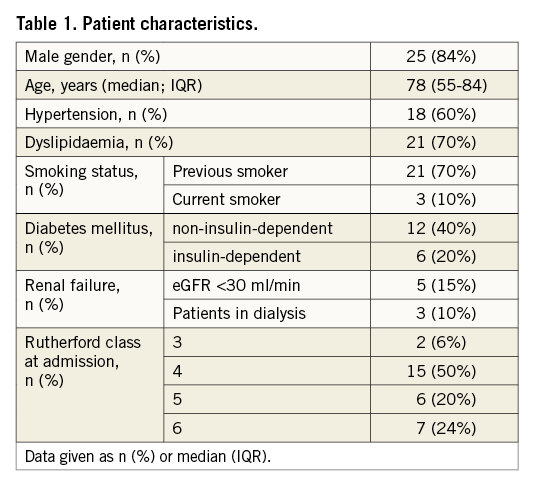
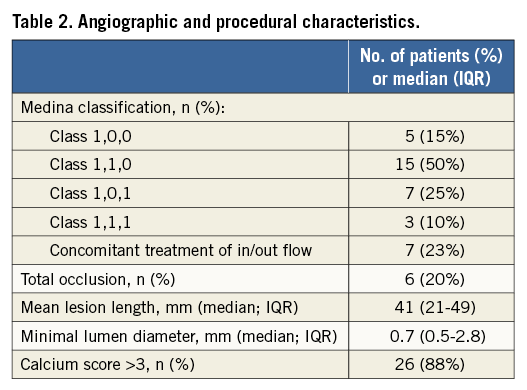
Out of a total of 30 cases, twenty cases (66%) underwent isolated CFA intervention, whereas 10 (37%) also received an inflow or outflow vessel intervention. Chronic total CFA occlusions (CTO) were recanalised in six cases (20%). In these cases balloon predilatation was performed before DA.
Success in crossing and debulking the target lesion was achieved in 100% (30/30) of cases with the mean diameter stenosis being reduced from 78±7% at baseline to 33±9% after DA, which was further reduced to 12.7±5.5% after adjunctive therapy with DCB. Technical and procedural success, defined as residual stenosis <30% at the end of procedure, was achieved in all patients, and no procedure-related adverse events occurred (including abrupt closures, vessel closure, distal embolisations and perforations) (Figure 1).
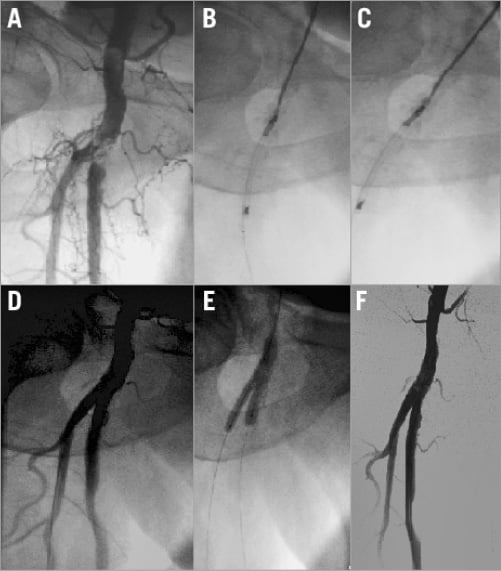
Figure 1. Treatment of complex common femoral artery obstruction using directional atherectomy and drug-coated balloon. A) Selective angiography showing a calcific obstruction of the distal CFA involving the ostium of both the superficial femoral artery (SFA) and the profunda femoral artery (PFA). B) Use of directional atherectomy to debulk the segment CFA-SFA. C) Use of directional atherectomy to debulk the segment CFA-PFA. D) Selective angiography showing optimal plaque removal after directional atherectomy. E) Simultaneous drug-coated balloon dilation. F) Selective angiography showing final result.
Due to the occurrence of flow-limiting dissection, persisting after prolonged DCB dilatation (>5 min), bail-out stenting was necessary in three cases (10%): in one case a 7.0×60 mm LifeStent® (Bard Peripheral Vascular, Tempe, AZ, USA) was implanted, and in two cases a 7.0×40 mm Protege™ (Covidien [now Medtronic]). Debris was collected in the reservoir in all cases, but in only four of them was a significant amount of debris collected in the filter basket. No distal embolisation occurred. The mean procedural time was 78.5±33.2 minutes and mean fluoroscopic time was 36.5±10.0 minutes. Among in-hospital complications, one access-site-related bleeding event occurred (haematoma) which was treated with manual compression (0.3%).
As summarised in Table 3, during follow-up restenosis occurred in two patients six months after the index procedure and in one patient at 11 months after the index procedure. In-stent restenosis occurred in one of the patients treated with bail-out stenting. Consequently, the primary duplex-documented patency rate at one year was 90.0% (27/30 lesions). The clinically driven TLR rate at 12 months was 6.7% (2/30 patients) and the primary endpoint (freedom from TLR) was achieved in 93.4%. In detail, two TLR were performed in two patients symptomatic for early onset claudication; in one patient, despite the detection of restenosis by duplex assessment, no TLR was performed due to the lack of symptoms.
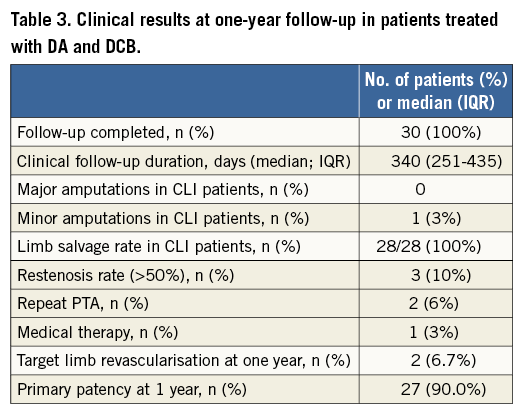
The Medina class of lesion and consequent PTA technique did not affect revascularisation rates.
At one year, mean ABI was 0.82±0.06 (baseline 0.49±0.11, p<0.05) and improved in all patients, by at least 0.1, during the period from baseline to 30 days post procedure.
Clinical success, based on improvement in the Rutherford classification at 12 months (1.8±1.6), compared to baseline 4.8±1.1 (p<0.05), was achieved in all patients. No major amputation was necessary, with a limb salvage rate of 100%, independently from the patients’ clinical presentation (LLC or CLI). In one case minor amputations were necessary to allow wound sealing and to preserve the patient’s ambulation. The rate of freedom from MAE at one year was 93.4% (28/30).
Discussion
This study suggests that the combined use of DA and DCB for the endovascular treatment of CFA obstructive disease is feasible and associated with good clinical outcome at one year.
The Transatlantic Inter-Society Consensus (TASC) II guidelines, published in 2007, recommend a surgical approach for CFA stenosis1. Surgical treatment of the CFA is associated with a technical success rate close to 100% and a favourable long-term outcome with cumulative patency rates up to 90% at five years2. However, the morbidity associated with the surgery (major haematoma, wound infection, nerve damage with persistent sensory disturbances) is not negligible. The need for surgical revision may occur in up to 5% of cases, and the incidence of minor complications (seromas and haematomas) may reach 20%12-14.
Thanks to modern endovascular equipment and techniques, the successful treatment of a growing number of TASC II type D lesions is now possible15,16. The largest data set of consecutive endovascular procedures involving severe atherosclerotic stenosis of the CFA available in the current literature shows that CFA percutaneous interventions are associated with high success, low complication rates (1.4% major and 5.0% minor complications) and a good one-year binary restenosis rate17.
In this registry, the technical procedural success with angioplasty alone, using balloons well matched to the original CFA vessel diameter, was achieved in 63.1% of the procedures. In the remaining cases where stenting was necessary, the investigators adopted a one-stent technique in order to avoid too many stents and strut overlap in the CFA bifurcation. Stenting resulted in being a favourable independent predictor for less restenosis and TLR at one year17. In the same study, the use of directional atherectomy in the treatment of non-calcific CFA lesions was also evaluated; even though not statistically significant, the use of DA was associated with a positive trend towards less TLR at one year17.
It has been well demonstrated that the use of DCB reduces restenosis occurrence in the treatment of femoropopliteal lesions18. In this registry, we report the results at one year of an endovascular strategy for the treatment of calcific CFA lesion, which combines the use of DA and DCB. In particular, the use of an atherectomy device, specifically dedicated to calcified lesions, allowed obtaining a proper lumen enlargement and minimising the occurrence of flow-limiting dissections, even when adjunctive PTA was used, thus limiting the need for stent implantation. This approach could be used in most of the possible CFA lesions (±SFA and/or PFA involvement).
The sustained clinical benefit at one year could be related to the use of DCB in all patients. Finally, our study proposes a combined approach, using DA and DCB, for the endovascular treatment of CFA lesions, a hypothesis that should be tested in a properly sized RCT.
A possible downside of this combined endovascular approach is device cost and the longer procedural time required for using the atherectomy device. This is related to the need for repeated catheter removal/reintroductions and the necessity to empty the reservoir when filled with atherosclerotic debris.
Moreover, it has to be borne in mind that the amount of debulking that can be done with atherectomy, particularly in large calcified eccentric plaque, is not comparable to that which can be done with a femoral endarterectomy. This may have implications in the long term.
Limitations
The main limitation of this study is the small sample size and the lack of a control group. Thus, it could be considered only a hypothesis-generating study. It provides data that could help the design of a future trial aimed at defining the appropriate endovascular therapy for those patients presenting with calcific lesions of the CFA.
Conclusions
This single-centre prospective study proposes the combined use of DA and DCB as a safe and effective endovascular therapy for the treatment of common femoral artery lesions. This hypothesis should be confirmed in a larger registry and possibly tested in a randomised trial of comparison with standard surgical therapy.
| Impact on daily practice The use of DA in the treatment of CFA lesions improves acute success, by debulking the fibrocalcific portion of the atherosclerotic plaque, but does not affect long-term patency rates. The use of DCB improved long-term patency rates after SFA PTA. This study, by demonstrating that the combined use of DA and DCB for the endovascular treatment of CFA obstructive disease is associated with good clinical outcome at one year, provides an important therapeutic strategy for the treatment of calcific common femoral artery occlusions in daily practice. |
Conflict of interest statement
The authors have no conflicts of interest to declare.

