Abstract
Aims: Endovascular treatment of calcified femoral-popliteal disease is challenging. We sought to evaluate the mechanism of lumen gain when using the JETSTREAM Atherectomy System to treat calcified peripheral artery lesions.
Methods and results: The JETSTREAM Calcium Study was a prospective, single-arm, multicentre study to evaluate the JETSTREAM Atherectomy System for severely calcified femoral-popliteal artery lesions, i.e., patients with claudication and lesions with superficial calcium >90° and >5 mm in length as determined by intravascular ultrasound (IVUS). The 2.1 mm catheter was used in this study without distal protection. Fifty-five patients underwent angiographic screening: 26 (45%) met IVUS inclusion criteria. Angiographic calcium was moderate in eight cases and severe in 14, with no available data for four cases. Visual diameter stenosis was 86±9% pre-treatment, 37±13% post atherectomy, and 10±6% post adjunctive treatment (adjunctive PTA+stenting in eight and adjunct PTA alone in 16). IVUS showed lumen area increased from 6.6±3.7 mm2 to 10.0±3.6 mm2 (p=0.001): calcium reduction was responsible for 86±23% of the lumen increase. Although the superficial calcium arc did not change (151±70° to 146±71°, p=0.83), the arc of reverberation increased (23±20° to 65±40°, p=0.006), indicating device-related modification of calcium. Adjunctive balloon angioplasty was performed in 62% of the lesions, and stent implantation in 31%. In 11 cases with adjunctive balloon dilation, the MLA increased from 7.1 (6.4, 7.8) mm2 post atherectomy to 11.9 (10.3, 13.5) mm2 post balloon (p<0.001) without flow-limiting dissection. No major adverse events occurred up to 30 days post procedure in either the study group or the patients who were excluded from the analysis.
Conclusions: The JETSTREAM Atherectomy System increased lumen dimensions in moderately or severely calcified femoral-popliteal lesions by removing superficial calcium without major complications.
Introduction
Endovascular treatment of the femoral-popliteal segment remains challenging with respect to restenosis. Walking and stair climbing may cause mechanical stent deformation including stent fracture. Even with the use of drug-eluting stents, the restenosis rate is as high as 20-30%, with 0-1.5% stent fracture1,2.
The JETSTREAM® Atherectomy System (Boston Scientific, Marlborough, MA, USA) is a peripheral revascularisation platform designed to actively remove atherosclerotic plaque, debris, and thrombus from stenotic peripheral arteries. Previous studies of lesions with angiographic moderate to severe calcification have shown a high acute success rate with an acceptable rate of clinically driven target lesion revascularisation (TLR) of 26% at 12 months3,4. However, the mechanism of lumen gain when using the JETSTREAM Atherectomy System to treat calcified lesions is unknown5. We hypothesised that the JETSTREAM Atherectomy System can debulk calcification without significant vessel injury. Therefore, we conducted a prospective study to assess calcium reduction.
Methods
STUDY DESIGN AND ENDPOINTS
The JETSTREAM Calcium Study was a prospective, single-arm, multicentre study to evaluate the effect of the JETSTREAM Atherectomy System when treating severely calcified peripheral arterial lesions defined as symptomatic lesions with superficial calcium >90° and >5 mm in length as evaluated by intravascular ultrasound (IVUS). The primary endpoint was calcium removal and luminal gain measured by comparing pre-intervention to post-atherectomy IVUS images. Secondary endpoints included: 1) major adverse events, defined as death, myocardial infarction, amputation, and target lesion or vessel revascularisation up to 30 days; 2) adjunctive therapy use; and 3) residual diameter stenosis. The inclusion criteria were: 1) claudication due to a stenosis in the common femoral, superficial femoral, or popliteal arteries requiring revascularisation; 2) proximal reference lumen ≥3.0 mm in diameter; and 3) moderate to severe obstructive intraluminal calcification as screened by angiography6 and confirmed by IVUS (superficial calcium >90° and >5 mm in length)7. The exclusion criteria were: 1) uncontrollable allergy to nitinol, stainless steel, or other stent material or contrast agent; 2) inability to take appropriate antiplatelet therapy; 3) no distal run-off vessels; 4) in-stent restenotic lesions; 5) moderate to severe angulation (>30°) or tortuosity at the treatment segment; and 6) a history of coagulopathy or a hypercoagulable bleeding disorder. The protocol was approved by the institutional review board at each hospital, and written informed consent was obtained from each patient.
Visual angiographic evaluation was carried out by the operator during the procedure. Angiographic calcification was defined as apparent densities noted within the vascular wall and considered as severe if calcification was >5 mm in length and located on both sides of the artery; otherwise, calcification was considered moderate. Diameter stenosis was estimated by comparing the minimum lumen diameter to the reference lumen diameter. Lesion length was evaluated as the length between the normal-looking proximal and distal reference sites.
The JETSTREAM Atherectomy System consists of a single-use catheter with control pod and a reusable, compact console that mounts to a standard I.V. stand. The catheter has a rotational, front-facing cutting tip that debulks both hard and soft plaque, as well as calcium, thrombus, and fibrotic tissue. The system provides active aspiration which continually removes excised stenotic material and thrombus from the treatment site, offers expandable blade technology, and is indicated for both atherectomy and thrombectomy. There are four catheters with cutter sizes ranging from 1.6 mm to 3.4 mm, of which the two larger diameter catheters have expandable blades that allow creation of two successive lumen sizes with a single catheter. The 2.1 mm catheter was used in this study. The size of the cutter in the blades down position is 2.1 mm, and the size of the cutter in the blades up position is 3.0 mm. The catheter is delivered to the treatment site over a compatible 0.014” guidewire. The catheter is advanced at a rate of 1.0 mm/s and pulled back slightly after each advance to allow the aspiration function to clear the area of any debris. The catheter drive motor provides audible feedback to help guide the procedure; slowing rotation changes the pitch of the motor and indicates that the cutter is encountering more difficult atheroma. Good technique requires a consistent maximum cutter tip speed so that the aspiration function is not overloaded and the cutter blades can work at maximum efficiency. Depending on the type of lesion and size of artery, the device may be passed through the blockage two or more times to achieve the full diameter of the cutter tip. Distal protection devices were not used in this study.
IVUS IMAGING PROTOCOL AND ANALYSIS
IVUS images were obtained before and after atherectomy. If adjunctive balloon angioplasty was performed, additional IVUS evaluation afterwards was encouraged. All IVUS studies were performed using a commercially available IVUS system (20 MHz Eagle Eye® catheter; Volcano Corp., Rancho Cordova, CA, USA). The IVUS catheter was advanced distal to the lesion, and imaging was performed retrogradely proximal to the lesion at an automatic pullback speed of 1.0 mm/s using an R-100 pullback device (Volcano Corp.). Images were recorded onto digital media for offline analysis at a single, independent core laboratory (Cardiovascular Research Foundation, New York, NY, USA) which was blinded to patient clinical outcomes.
Using computerised planimetry software (echoPlaque; INDEC Medical Systems, Santa Clara, CA, USA), the lumen borders were traced. Using vascular and perivascular landmarks and the known pullback speed, images pre and post atherectomy were matched and analysed side by side. The treated segment was identified and divided into sub-segments in relation to the existence of superficial calcium and its modification. By comparing pre- versus post-atherectomy IVUS images, we identified three slices, at least 5 mm apart, which showed the greatest calcium reduction. First, the pre-intervention and post-atherectomy lumens were contoured. Second, the post-atherectomy IVUS images were overlaid onto their respective pre-intervention images, and the reduction in lumen area to either calcified plaque or non-calcified plaque removal, assuming no change in total arterial area (Figure 1), could then be assessed.
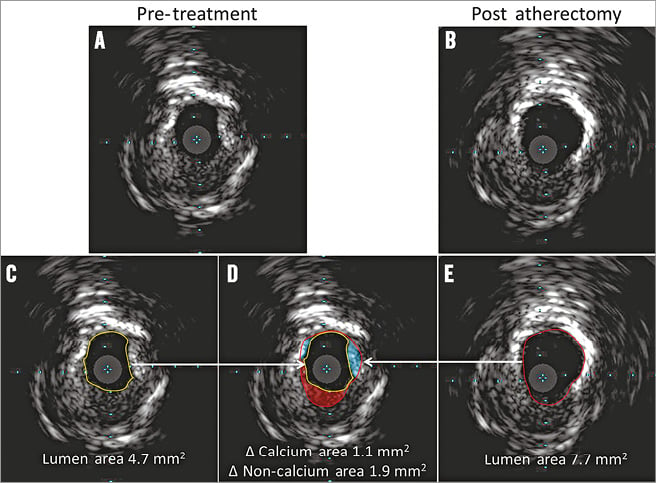
Figure 1. Method of IVUS analysis of calcium reduction. Pre-treatment IVUS (A), and the corresponding post-atherectomy IVUS (B). The analysis sequence is shown at the bottom. After identifying and matching the slices with calcium reduction, the lumen borders for both pre-treatment (yellow circle: lumen area=4.7 mm2) (C) and post-atherectomy images (red circle: lumen area=7.7 mm2 ) (E) were contoured; and the two were overlaid (D). By comparing the two contours to the visual assessment of plaque, lumen gain (3.0 mm2) could be attributed to a reduction of calcified plaque (blue area=1.1 mm2) or to a reduction of non-calcified plaque (red area=1.9 mm2).
Qualitative IVUS parameters included: 1) surface shape of calcification (convex or concave, irregular or smooth), 2) existence of reverberations (indicating device-related modification of calcium) and the angle of reverberation with respect to the centre of the lumen, and 3) presence of a dissection including the arc of the dissection flap and the minimum lumen area within the dissection (Figure 2).
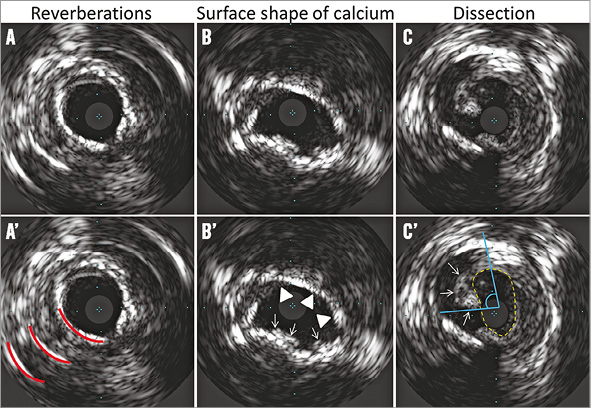
Figure 2. IVUS qualitative analysis. A-C correspond directly to A’-C’. A) Reverberations (red lines in A’) due to polished calcified plaque. B) Irregular convex-shaped superficial calcified plaque (white arrows in B’) and smooth concave-shaped superficial calcified plaque (white triangles in B’). C) Intimal dissection (white arrows in C’); the effective lumen area within the dissection flap measured 4.9 mm2 (yellow dotted area in C’) with arc of dissection of 89° (blue angle in C’).
STATISTICS
Statistical analysis was performed using SAS software, Version 9.1 (SAS Institute, Cary, NC, USA). Continuous variables were presented as mean and standard deviation or median (Q1, Q3) based on the distribution, and categorical variables were presented as frequencies. Continuous variables were compared using the Mann-Whitney U test. Categorical variables were compared using chi-square statistics. For calcified plaque, a model with generalised estimating equations (GEE) was used to compensate for any potential cluster effect of multiple calcified plaques in the same lesion, and the results were presented as least square means with 95% confidence intervals. A p-value <0.05 was considered significant.
Results
Between March 2011 and February 2012, 55 patients with angiographic moderate to severe calcification were enrolled and treated using the JETSTREAM Atherectomy System at five study centres in the USA (Figure 3). The pre- and post-procedural IVUS images were sent to the core lab: 26 patients met IVUS criteria (superficial severe calcification) and were formally analysed. Non-qualified IVUS images included non-calcified plaque at the stenotic segment with superficial calcium only at non-stenotic segments in 16 cases, deep calcification only in 11 cases, no post-treatment IVUS images in one case, and technical problems with the digital medium in one case. Among the 26 qualified cases, 64% were male, with a mean age of 73±11 years. Diabetes mellitus was present in 56% of the patients.
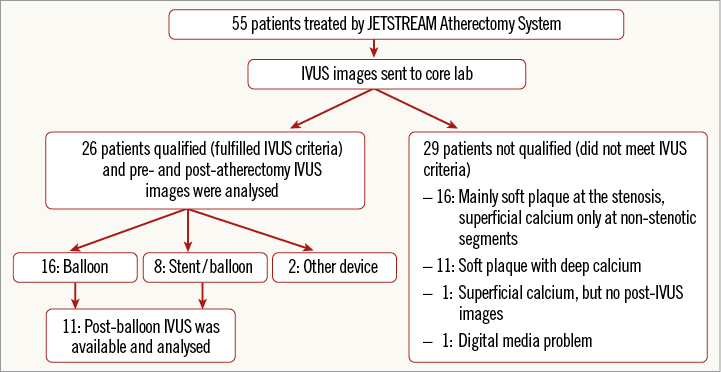
Figure 3. Diagram of patient flow. Fifty-five patients were enrolled based on angiographic criteria and 26 patients were qualified for analysis based on IVUS criteria.
As shown in Table 1, the majority of lesions were located in the superficial femoral artery: superficial femoral artery only in 15 cases, superficial femoral artery extending into the popliteal artery in three cases, superficial femoral artery extending into the common femoral artery in one case, and popliteal artery only in five cases. Lesion location was not specified in two cases; however, IVUS images were still available for analysis.
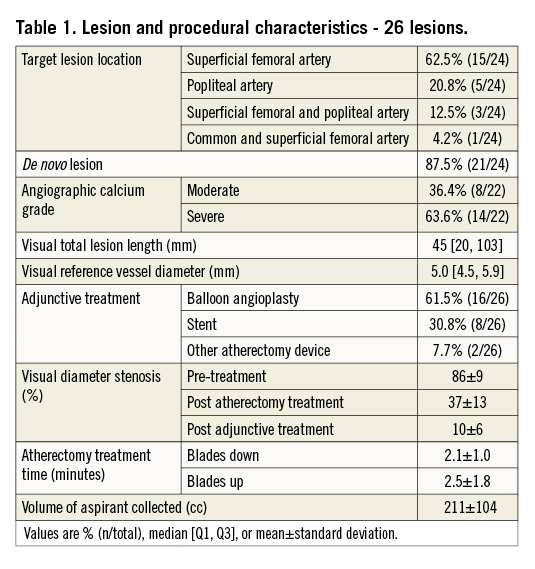
Angiographic calcium was moderate in eight cases and severe in 14, with no available data for four cases. The median vessel diameter was 5 mm (range: 2.8-12.5 mm), and the median lesion length was 45 mm (range: 5.2-350 mm). Adjunctive treatment after atherectomy included balloon angioplasty only in 16 cases, stent implantation in eight cases, another atherectomy device (TurboHawk™ LX-C; Covidien, Plymouth, MN, USA) post JETSTREAM atherectomy in one case, and use of the Crosser® catheter (Bard Peripheral Vascular, Inc., Tempe, AZ, USA) pre JETSTREAM atherectomy in one chronic total occlusion case. The visually assessed angiographic diameter stenosis improved from 86±9% pre-treatment to 37±13% post atherectomy and finally to 10±6% post adjunctive treatment. There were no angiographic differences comparing patients treated with JETSTREAM atherectomy plus balloon angioplasty vs. JETSTREAM atherectomy plus stent implantation. The mean treatment time was 2.1±1.0 minutes with the catheter in the “blades down” (unexpanded) configuration position, and 2.5±1.8 minutes in the “blades up” (expanded) configuration position. The total volume of aspirant collected was 211±104 cc.
After matching pre- and post-atherectomy IVUS segments, the treated segment median length measured 36 (21, 70) mm. The segment of superficial calcium with calcium reduction measured 13 (5, 20) mm in median length, or 37% (17, 57) of the entire treated segment.
As shown in Table 2, the IVUS findings from pre to post atherectomy at the lesion level showed that the minimum lumen area increased from 5.1±2.8 mm2 to 8.3±2.6 mm2 (p<0.0001), and area stenosis (1 - minimum lumen area/reference lumen area) decreased from 64±17% to 41±18% (p<0.0001). By comparing pre- and post-atherectomy IVUS images, the slice with the maximum calcium reduction was identified; the lumen area in these slices increased from 6.6±3.7 mm2 to 10.0±3.6 mm2 (p=0.001). The decrease in calcium area, measured as 2.8±1.6 mm2, was responsible for 86±23% of the lumen area increase.
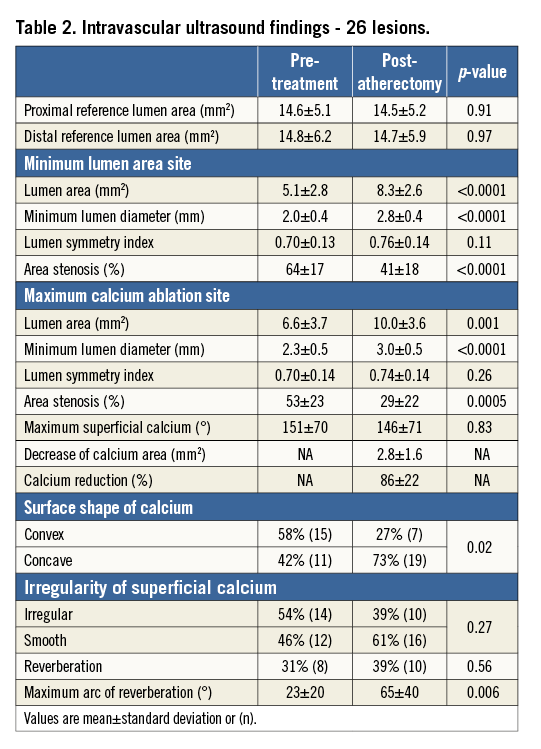
From the treated calcified segments, a total of 70 calcified plaques showing significant reduction of superficial calcium were selected. Three calcified plaques were identified in each of 21 lesions, while only two such plaques could be identified in two lesions and one plaque could be identified in the remaining three lesions. Pre- and post-atherectomy treatment comparisons of these plaques were made using GEE adjustment. As shown in Table 3, the lumen area increased from 6.4 mm2 to 9.6 mm2 (p<0.0001), with a 2.2 mm2 decrease in calcium that was responsible for 77% of the lumen area increase.
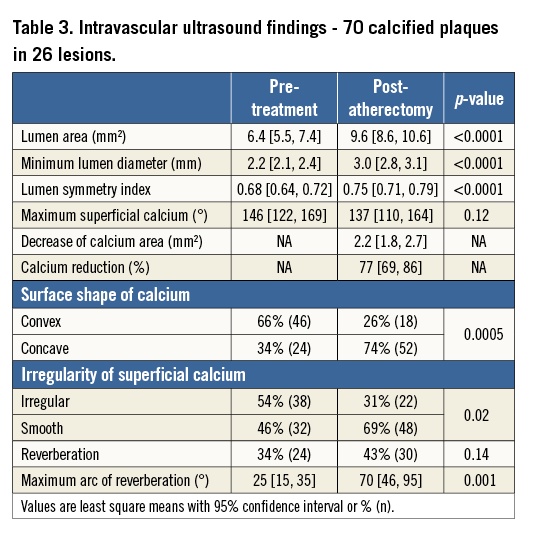
Although the arc of superficial calcium did not change significantly, the arc of reverberation increased from 25° to 70° (p=0.001). The prevalence of a convex shape and irregularity of the surface of calcium decreased from 66% to 26% and 54% to 31%, respectively, while the prevalence of a concave shape and a smooth surface of calcified plaque increased. A representative case is shown in Figure 4.
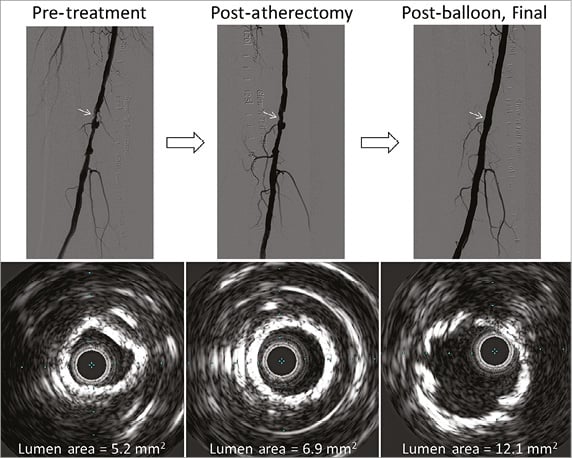
Figure 4. A representative case of pre-treatment, post-atherectomy, and post-balloon final images. The lumen increased from pre-treatment (5.2 mm2) to post-atherectomy (6.9 mm2) to post-balloon (12.1 mm2) without dissection.
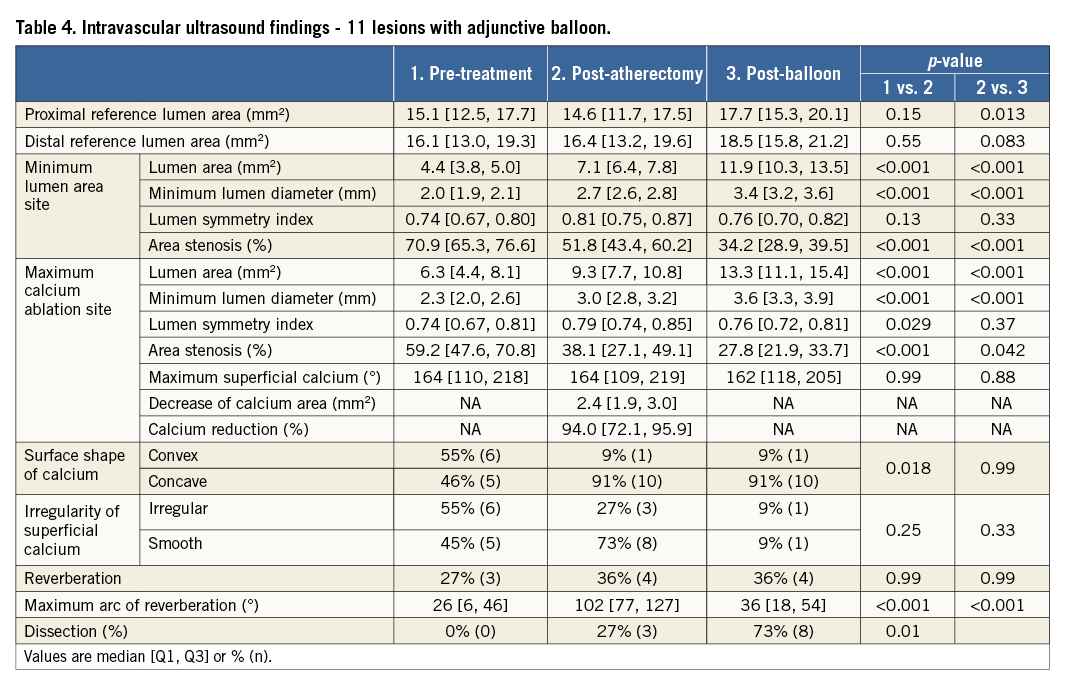
Table 4 shows the findings of 11 lesions which had post-adjunctive balloon IVUS images. The resulting lumen area was greater than that achieved with atherectomy alone; however, the prevalence of dissections increased (post atherectomy, 27% [3/11]; post balloon, 73% [8/11], p=0.025). The maximum angle of the dissection flap was minor (42° [17, 66]) with a preserved lumen area (15.6 mm2 [13.4, 17.7]) within the dissection.
No major adverse events occurred up to 30 days post procedure in either the study group or the patients who were treated but excluded from the analysis.
Discussion
The major findings are as follows: 1) the JETSTREAM System removed and modified moderate to severe superficial calcium to achieve significant lumen gain, and 2) adjunctive balloon angioplasty after calcium modification showed further lumen increase without major complications.
Earlier studies of the treatment of calcified lesions in atherosclerotic peripheral arteries were conducted with different debulking devices. These studies reported on clinical outcomes, but not on the characteristics of calcium removal itself. Zeller et al3 reported on 210 femoral-popliteal artery lesions treated using the JETSTREAM device. Moderate to severe calcium was present in 51% of lesions, of which 31% were total occlusions. One third of the procedures required no adjunctive therapy, and 59% of lesions needed adjunctive low-pressure (<8 atm) balloon angioplasty. Major adverse events at 30 days occurred in two patients (1%), and at one year 42 patients (26%) underwent clinically driven TLR. The need for adjunctive therapy was higher in our cohort than in this published study: this may be explained by the fact that our cohort included more severely calcified lesions as documented by IVUS and as required by the protocol. In the same cohort, Sixt et al4 reported a sustained quality of life improvement with an improved ankle-brachial index. However, the severity of calcium was not described in this report.
OTHER DEVICES
Other atherectomy devices have been used to treat calcified lesions. The Rotablator™ (Boston Scientific) is usually used in highly calcified infrapopliteal arteries resistant to balloon angioplasty8. The SilverHawk™ Plaque Excision System (Covidien) is a directional device which uses a rotating blade to excise plaque and pack the material into a distal storage chamber9,10. The data from the TALON registry included 601 patients with 1,258 lesions9. Moderate to severe calcification was present in 65% of the lesions, and 75% of the lesions were above the knee. Nearly three quarters (73%) of the lesions did not require adjunctive therapy, ankle-brachial index increased from 0.70±0.23 pre-treatment to 0.86±0.20 post treatment, and the rate of TLR at 12 months was 20%. The recently published DEFINITIVE Ca++ trial further confirmed the efficacy of SilverHawk and TurboHawk (specially designed for calcific plaque) used together with distal embolic protection11. A total of 133 subjects with 168 moderate-to-severely calcified femoropopliteal artery lesions were enrolled and achieved the primary effectiveness endpoint (diameter stenosis ≤50%) in 92% with only 4.1% (7/169) bail-out stent usage and 6.9% experiencing a major adverse event at 30 days.
Lastly, the Orbital Atherectomy System (OAS) (Cardiovascular Systems, Inc., St. Paul, MN, USA) has a diamond-coated abrasive crown (Diamondback®) which sands calcified and fibrotic plaques during high-speed rotation12,13. Korabathina et al13 reported on 200 lesions treated by OAS: 78% of these lesions were calcified, and 59% were located in the femoral artery. The rate of procedural success (residual stenosis ≤30%) was 86%, and adjunctive therapy was needed in 68%. Shammas et al12 reported on 50 patients randomised between OAS plus percutaneous transluminal angioplasty (PTA) versus PTA only. A lower target vessel revascularisation rate was found in the OAS plus PTA group (6.7%) compared to the PTA only group (20%) at 12 months.
The sensitivity of calcium detection by IVUS relative to pathology has been reported as 89-90% with a specificity of 97-100%7,14,15. Regarding the difference between angiography and IVUS, Mintz et al7 reported that IVUS detected calcium in 841 of 1,155 coronary artery lesions (73%), while angiography detected calcium in only 440 (38%). In their report, the overall sensitivity of angiography relative to IVUS was 48% with a specificity of 89%. Furthermore, IVUS can differentiate between deep and superficial calcification; this is difficult to determine by angiography. Therefore, to confirm the true efficacy of calcium reduction, IVUS was used to identify and evaluate calcified lesions in the JETSTREAM Calcium Study. Only severely superficial calcified lesions were included in this study. Half of the lesions identified angiographically with moderate to severe calcification had no superficial severe calcification as determined by IVUS. These lesions were excluded from the final analysis. In previous studies the incidence of angiographic dissections was reported as 14.0-17.1% after conventional balloon angioplasty12,16, 3.4-15.5% after OAS12,13,17, 9% after JETSTREAM atherectomy3,4, and 3.6% after SilverHawk atherectomy9,11. This was reflected by the frequency with which adjunct stenting was performed. While the current study showed a higher rate of dissections, they were relatively minor non-flow-limiting dissections due to higher resolution of IVUS versus angiography. However, the requirement of stent usage (30.7%, 8/26) was relatively high, perhaps because our cohort all had IVUS-confirmed severe superficial calcification.
Severely calcified lesions may cause damage to the polymer/drug coating of a drug-eluting stent, resulting in inadequate drug delivery18,19. Although there is accumulating evidence in coronary artery intervention showing that calcified lesions have worse outcomes compared to non-calcified lesions20,21, the clinical impact of superficial calcium removal in peripheral artery disease in respect of effectiveness of drug-coated balloons or drug-eluting stents needs further investigation22.
STUDY LIMITATIONS
The number of cases which finally qualified for inclusion was relatively small. However, this study was designed to evaluate the mechanism of calcium removal and not clinical outcomes. In addition, quantification of calcium removal was limited in that calcium thickness cannot be determined by IVUS.
Conclusions
The JETSTREAM Atherectomy System removed and modified superficial calcium to achieve significant lumen gain. Adjunctive balloon angioplasty after calcium modification showed further lumen increase without major complications.
| Impact on daily practice The JETSTREAM Atherectomy System increased lumen dimensions in moderately or severely calcified femoral-popliteal lesions by removing superficial calcium. Furthermore, this modification of superficial calcium facilitated further lumen expansion during adjunctive balloon post-dilation without major dissections. In daily practice, the JETSTREAM Atherectomy System has potential as an adjunctive device for peripheral percutaneous intervention. |
Funding
This study was supported by Bayer HealthCare.
Conflict of interest statement
A. Maehara receives a grant from and is a consultant to Boston Scientific. G. Mintz receives grant support and is a consultant for Boston Scientific and Volcano Corporation. W. Gray is a consultant to Bayer HealthCare. T. Davis is a consultant to Bayer and has given training courses for Bayer. M. Foster has received speaker’s honoraria from Volcano Corporation. T. Shimshak is a consultant for Bayer HealthCare, Boston Scientific, and Volcano Corporation. The other authors have no conflicts of interest to declare.

