Case summary
Background: An 80-year-old man with limiting angina pectoris.
Investigation: Physical examination, laboratory tests, echocardiography, exercise ECG, coronary arteriography, pressure wire assessment.
Diagnosis: Single severe calcific coronary artery disease.
Treatment: Elective percutaneous coronary intervention (PCI) for calcific mid-vessel stenosis with rotational and excimer laser atherectomy.
Keywords: Excimer laser, stent underexpansion, percutaneous coronary intervention.
Presentation of the case
An 80-year-old man with angina pectoris, significant left anterior descending (LAD) disease on fractional flow reserve (FFR) underwent elective percutaneous coronary intervention (PCI) for calcific mid-vessel stenosis (Figure 1A, Moving image 1). Rotational atherectomy was undertaken using 1.75mm and 2.0mm burrs (Boston Scientific, Natick, MA, USA) with successful debulking and polishing runs (Moving image 2). Despite this, there was failure of complete balloon expansion on predilatation (3.0×20mm Maverick® [Boston Scientific] at 16 atm). Therefore, further lesion preparation was performed with a variety of balloons without success, despite high inflation pressures (3.0×20mm Voyager™ non-compliant [NC] balloon; Abbott Vascular, Rangendingen, Germany), 3.0×10mm Flextome® Cutting balloon® (Boston Scientific) and a 3.25×20 mm Voyager™ non-compliant balloon (Figure 1B). Intravascular ultrasound (IVUS, Eagle Eye®, Volcano, San Diego, CA, USA) revealed a concentric ring of calcification with a minimal luminal area (MLA) of 2.8 mm2 within the short segment of lesion that remained non-dilatable (Figure 2).
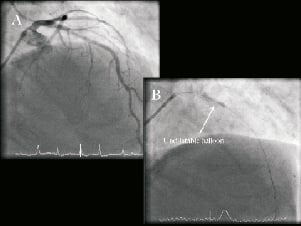
Figure 1. Diagnostic angiogram: A. Posterior anterior (PA) cranial view of left coronary artery (LCA) demonstrating the severe calcific stenosis in the mid left anterior descending artery (LAD). B. Failure of balloon expansion, despite aggressive preparation with rotational atherectomy and cutting balloon.
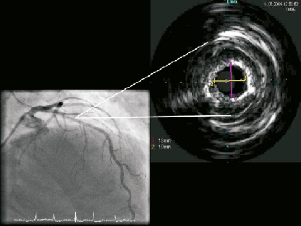
Figure 2. PA cranial view of LAD, demonstrating the concentric ring of calcium on angiography and intravascular ultrasound (IVUS).
In view of the vessel barotrauma, two TITAN 2 (HEXACATH, Rueil-Malmaison, France) overlapping stents were deployed in the mid-LAD (3.5×22 mm distally and 3.5×13 mm proximally) to permit safer high pressure balloon inflation dilatation. It was reasoned that stenting the segment would permit controlled high pressure balloon inflation to dilate the non-expanded lesion. There was angiographic stent underexpansion in the mid-segment despite post-dilatation with 3.5×20 mm and 3.75×20 mm Voyager NC balloons at 26 atm (Figure 3, Moving image 3). The intravascular ultrasound appearance remained unchanged despite using a double-coated OPN NC® 3.010mm Schwager balloon (Schwager Medica, Winterhur, Switzerland) inflated to over 40 atm (Moving image 4). At this point we elected to stop the procedure.
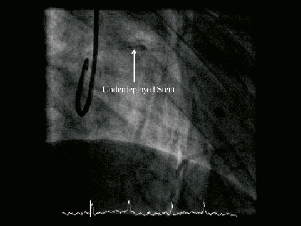
Figure 3. Fluoroscopic image demonstrating the stent, clearly underdeployed in the proximal third.
Conflict of interest statement
Drs. O’Kane and Talwar are proctors for Spectranetics Inc., Colorado Springs, CO, USA. The other authors have no conflicts of interest to declare.
How would I treat?
The Invited Experts’ opinion
In this case an 80-year-old man with angina pectoris underwent elective percutaneous coronary intervention (PCI) for significant FFR-positive calcific mid-vessel LAD stenosis. Despite extensive and successful debulking measures using rotational atherectomy (up to 2.0 burr), a remaining concentric ring of calcification could not be dilated either by the use of the various non-compliant high-pressure balloons, or by the use of a 3.0/10 mm Flextome cutting balloon. In order to apply even higher pressures more safely, two TITAN 2 stents have unfortunately been implanted subsequently with overlap. Not unexpectedly, the consecutive stent under-expansion remained despite post-dilatations, with numerous high pressure balloons inflated over 40 atm.
Although interventionists have to face this “unhappy double” (that is, an undilatable lesion plus an implanted steel “framework”) only occasionally after direct stenting in unexpected fibrotic lesions, this unhappy event of a resistant undilatable lesion was predictable, and treatment options were significantly reduced by the implanting of two stents on the basis of the relatively precarious idea that higher pressures can be applied more safely when a stent has been implanted in this very resistant lesion.
In order not to leave the case like it is, or to improve the chance of restenosis by just introducing a drug-eluting balloon (DEB) or (DES) stent (which is apparently one option in an 80-year-old man, where life expectancy may be lower than the risk of prognosis relevant restenosis, and where the various interventional options may include unpredictable risks in addition to the fact that surgical revascularisation is a realistic alternative1), there are theoretically only two interventional options left: 1) get rid of the additional metallic implant in this very resistant lesion by the use of an ablating technique (rotational or mechanical atherectomy) and, 2) to find ways to weaken the underlying restrictive lesion through the implanted stent, in order to make it more suitable for adequate stent expansion.
Rotational atherectomy is time-consuming, not always successful, and a very complex option to disperse the stent struts as well as the remaining underlying tissue. This is mainly based on personal experience, non-published communications and a small number of published case reports1.
Considering the use of the Silverhawk atherectomy system in coronaries comes out of the era where we treated diffuse in-stent restenosis with debulking techniques. The first and second generation Silverhawk atherectomy device for coronary use (SilverHawk® Plaque Excision System; EV3 Inc., Plymouth, MN, USA) ablated easily –not only the hyperplastic neointima of the in-stent restenosis– but also large amounts of the underlying stent. Stent debris was frequently found in the ablated material. Possibly, on basis of occasionally blocked knifes in stent struts, this approach has never drawn exceptional attention. Due to these same reasons, it was also unsafe to use directional atherectomy (Flexi-cut Directional Debulking System, Abbott Laboratories, Abbott Park, IL, USA) for in-stent restenosis.
The only alternative to debulking techniques is the option to weaken or ablate the underlying restrictive lesion through the implanted stent endovascularly, and then, afterwards, to adequately post-dilate the implanted stent. In this perspective, radiofrequency and laser ablation are the only endovascular techniques so far which have shown ablative capabilities.
The Safe Cross-RF guidewire (Intraluminal Therapeutics, Carlsbad, CA, USA), a steerable 0.014-inch intermediate-stiffness guidewire, measures not only the reflection of near-infrared light (optical coherence reflectometry) ahead of the wire tip for guidance in chronic occlusions, but can also deliver radiofrequency energy for tissue ablation, and, therefore, can be used for crossing atherosclerotic occlusions2,3. I have no personal experience or communications on this subject, neither are published data available describing the use of radiofrequency ablation in a case like this, but theoretically it may help to modify the underlying plaque for subsequent postdilatation of the implanted stent.
Excimer lasers emitting 308nm have the capability of concomitantly ablating atherosclerotic plaque. The ablative capabilities of the laser are based on absorption of its energy in the atheroma, leading to photomechanical and photothermal processes2,3. Using high power energy, the 0.9 X-80 catheter (Spectranetics Corp., Colorado Springs, CO, USA) have shown to cross even heavily calcified lesions2. Even if the target lesions are not directly in the focus of laser beam, the specific interaction of excimer laser energy in blood vessels includes induction of acoustic shock waves propagating onto the surrounding structures, which is exactly the desired objective in this case. Based on my personal experience of laser-effects underneath an implanted stent –which is also comes out of an era when laser techniques were used to ablate neointima in diffuse in-stent restenosis– the laser obviously ablated tissue underneath the stent in cases where the laser catheter got trapped in stent struts, and a relatively greater amount of laser energy was applied at the same place2,3. Taking this undesirable side effect as a challenge, Ibelieve, that using a laser for this restrictive and superficially stented lesion may modify the lesion considerably beneath the stent, and make it potentially suitable for full stent expansion.
In conclusion, interventional options to improve the local morphology theoretically exist, but are based on anecdotal and personal experiences or communications without proof, and include the risk of vessel wall perforation. The “second-best” interventional solution of lowering the probability of restenosis through the use of anti-proliferative drugs (DEB, DES) or surgical revascularisation (i.e., when using minimal invasive techniques), are possibly better options in a 80-year-old man, where life expectancy may be lower than the risk of prognosis relevant recurrent stenosis.
Conflict of interest statement
Dr. Dahm is a member of various Advisory Boards (Boston Scientific Europe, Biotronik and Abbott).
How would I treat?
The Invited Experts’ opinion
The authors describe a typical case of stent under-expansion in the mid-segment of a very calcific left anterior descending (LAD). The lesion was prepared with a rotational atherectomy after failing to predilate with a non-compliant (NC) balloon and with a cutting balloon. In our experience, these types of resistant lesions are rare, but when they occur they are very laborious to treat. Stent under-expansion may lead to in-stent restenosis and to an increased risk of stent thrombosis1. As a general approach, we prefer not to implant a stent unless we are sure we obtained full balloon expansion with predilatation. If we still notice balloon indentation, we repeat rotational atherectomy utilising a larger burr.
In the case presented here the operator decided to implant a stent despite suboptimal balloon dilatation and then try further inflations with higher pressure balloons. Despite these attempts the stent could not be fully expanded. Presently, we utilise an Excimer Laser and concomitant contrast injection to solve the problem of stent underexpansion as described by Goldberg et al2. In the last year, we treated four patients with stent underexpansion, and recurrent restenosis with an excimer Laser with good final results and without procedural complications. We report a case of in-stent restenosis occurring three months after suboptimal stent implantation in a calcific lesion of the left anterior descending artery, which could not be dilated despite repeated and prolonged high-pressure balloon inflations (Figure 4). Finally, we decided to utilise a 1.4 mm high-energy excimer laser coronary atherectomy catheter, (Spectranetics, Colorado Springs, CO, USA). This 6Fr compatible catheter is able to deliver excimer energy from 30 to 80mJ/mm at pulse repetition from 25 to 80hertz. We performed laser angioplasty utilising the following parameters: 40mJ/mm (max fluence) and 80Hz (max repetition rate for 20 seconds) without applying the classical “flush and bathe” technique, which is usually used to replace blood and contrast within the vessel with saline before activating the laser to decrease vapour bubble formation. On the contrary, we lased with simultaneous contrast injection taking advantage of the acoustic-mechanical trauma to alter the underlying lesion morphology, to facilitate lesions dilatation and achieve full stent apposition. Another possible technique is to use rotational atherectomy to treat the underexpanded stent. However, we rarely perform rotational atherectomy in this context. Nevertheless, rotational atherectomy has been described as a possible treatment for stent underexpansion3.
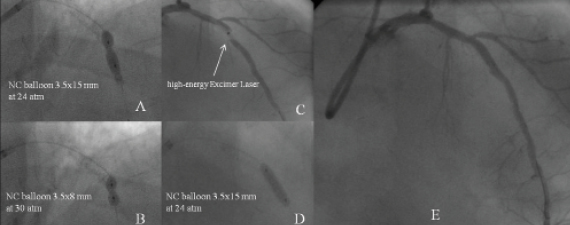
Figure 4. A case of in-stent restenosis in a calcific lesion of the left anterior descending artery, which could not be dilated despite repeated and prolonged high-pressure balloon inflations (A,B), utilisation of a excimer laser coronary atherectomy catheter (C) , post-dilation ( D) and final result (E).
Conflict of interest statement
The authors have no conflicts of interest to declare.
How did I treat?
Actual treatment and management of the case
The patient was electively re-admitted 10 days later for ELCA (CVX-300®; Spectranetics, Colorado Springs, CO, USA) to the segment of stent under-deployment (Moving image 5). IVUS re-examination revealed significant neo-intimal hyperplasia at the point of underexpansion with a MLA of only 2.5mm2 (Figure 5). A 2.0 mm excimer laser catheter was used initially delivering 20 trains (4,500pulses) at 60mJ/mm2 fluence/40Hertz. The 2.0mm catheter would not fully traverse the stent, and a 0.9 mm catheter was subsequently used to deliver a further 12 trains (5,000 pulses) at 80mJ/mm2 fluence/80Hertz. Following ELCA, a 3.7520 mm Voyager NC balloon dilated fully at 18 atm, with angiographic and IVUS confirmation of complete stent expansion (Figure 6, Moving images 6 and 7).
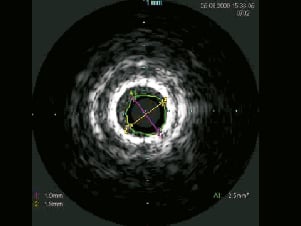
Figure 5. IVUS at 10-days post stenting demonstrating the underdeployed stent with significant early neo-intimal hyperplasia.
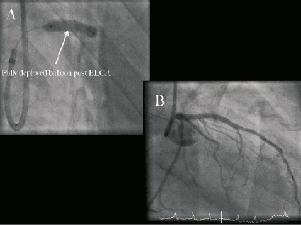
Figure 6. Right anterior oblique (RAO) caudal angiographic images showing complete balloon expansion (A) and the final angiographic appearance (B) following excimer laser coronary atherectomy (ELCA).
Discussion
Calcified lesions may be resistant to balloon and stent expansion, which significantly increases the subsequent risks of in-stent restenosis (ISR) and stent thrombosis.
We describe a case of stent underexpansion despite aggressive lesion preparation with rotational atherectomy; cutting, non-compliant and double-coated balloons, in which excimer laser coronary atherectomy (ELCA®) allowed subsequent, complete stent expansion.
Laser atherectomy may be useful in the management of stent under-expansion. The case described here is the first reported demonstration of the effectiveness of ELCA under intracoronary saline infusion to permit full stent expansion in a heavily calcified lesion that had been prepared with rotational atherectomy.
The adjunctive technique of ELCA has many applications, including the treatment of non-dilatable and/or calcified lesions, acute thrombus ablation and in debulking saphenous venous grafts1. ELCA utilises a xenon chloride gaseous medium, which generates ultraviolet light energy with a wavelength of 308 nm and a shallow penetration depth of only 50µm3. ELCA catheters can be delivered to the lesion on conventional 0.014” angioplasty guidewires and are activated with slow advancement (0.5-1.0mms–1) during intracoronary saline infusion to minimise vessel dissection and perforation4.
There are a number of studies and registries that demonstrate high procedural success and low complications with this technology for selective indications5-7. However, the use of ELCA for underexpanded stents has only previously been reported in one case, where a stent was successfully expanded five months after implantation. In this patient ELCA was used in the absence of saline infusion in order to potentiate the energy8. As demonstrated, conventional balloon therapies for underexpanded stents in this situation are limited9, and the role for ELCA represents a potential new indication. The likely mechanism of action is the disruption of tissue deep within the calcific ring, effectively weakening this structure and enabling full balloon expansion. The successful use of ELCA in this case avoided the need to consider surgical revascularisation, which may well have been indicated given the early target vessel failure.
Conflict of interest statement
Drs. O’Kane and Talwar are proctors for Spectranetics Inc., Colorado Springs, CO, USA. The other authors have no conflicts of interest to declare.
Online data supplement
Moving image 1. Coronary angiography pre-rotablation.
Moving image 2. Coronary angiography post rotablation with 1.75 and 2.0 burrs.
Moving images 3 & 4. Coronary angiography and IVUS demonstrating underexpanded stent in the calcified LAD.
Moving image 5. Coronary angiography pre-ELCA 10 days after the first procedure.
Moving images 6 & 7. Angiographic and IVUS demonstration of complete stent expansion.

