CASE SUMMARY
BACKGROUND: A 72-year-old male patient with previous CABG complaining of persistent NYHA stage 3 angina after incomplete percutaneous myocardial revascularisation caused by stent non-deployment in a heavily calcified proximal left anterior descending artery.
INVESTIGATION: Cardiac catheterisation.
DIAGNOSIS: Proximal LAD lesion stent underdeployment.
MANAGEMENT: Staged PCI.
KEYWORDS: complex angioplasty, coronary artery disease, rotablation, undeployed stent
PRESENTATION OF THE CASE
A 72-year-old hypertensive patient was admitted with severe angina complaints. His past medical history revealed prostatic cancer treated by surgery and radiotherapy and previous single venous bypass grafting to the right coronary artery (RCA) 20 years before. Two months earlier, angiography had been performed in another hospital for stable angina, demonstrating an occluded venous graft to the RCA, chronic occlusion of the native vessel and type II collaterals from the left artery. A critical heavily calcified lesion of the proximal LAD was identified as the culprit lesion and treated. Despite failure to predilate the vessel adequately, a 3×18 mm XIENCE V® stent (Abbott Vascular, Santa Clara, CA, USA) was positioned at the lesion site but remained undeployed or underdeployed in its middle part. Several further attempts to open the stent with high-pressure non-compliant balloons failed and the procedure was abandoned. Redo surgery was not considered and medical therapy was increased. Because of persistent and incapacitating symptoms, the patient was referred to our institution, where repeat angiography confirmed the previous findings and the presence of a second, angiographically significant lesion in the proximal LAD (Figure 1A, Figure 1B). The patient was discussed again within the Heart Team.
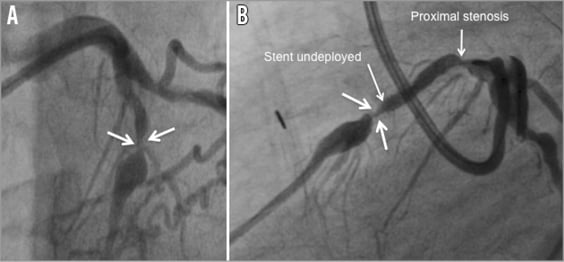
Figure 1. Coronary angiogram (Bi-plan). A) Image of the undeployed stent in the frontal view. B) Image of the non-deployed stent in the lateral view.
How would I treat?
THE INVITED EXPERTS’ OPINION
This is a patient presenting with an avoidable complication of stenting. Despite the inability to expand a balloon fully in a calcified lesion, stenting was attempted and, as expected, resulted in underdeployment. Despite few reports on intensified medical therapy of underdeployed/embolised stents1,2, we should not discharge this patient without solving the problem. The unstable situation in the proximal LAD together with RCA CTO with left to right collaterals is potentially very dangerous. Stent thrombosis would most likely lead to a catastrophic event, such as profound cardiogenic shock or cardiac arrest. Last but not least, symptoms remained and became even worse because of an undeployed stent in the tight LAD stenosis and an additional proximal LAD lesion. If not overlooked during the index procedure, it might be the result of vessel barotrauma or deep seating of the guiding catheter.
We would first discuss the situation within the Heart Team. Our suggestion would be CABG with LIMA to LAD and RIMA to RCA. Except for prostatic cancer, which seems to be under control, and the risks involved in a redo CABG, there are no other significant limiting comorbidities. If CABG, for whatever the reason, cannot be realised, an attempt should be made to solve LAD percutaneously.
Since the stent was already post-dilated, retrieval with a two-wire technique3, with guidewire and distal occlusion balloon4 or snares, would probably not be successful and is also potentially dangerous. A novel therapeutic option would be to advance a xenon-chloride pulsed-wave excimer laser catheter (ELCA) capable of delivering higher energy density with low heat production which has been demonstrated to be useful in balloon-resistant calcified stenosis5. ELCA in combination with contrast injection causes cavitation of the calcified plaque behind the stent struts, thereby facilitating stent expansion. Another option would be “stent and plaque” atherectomy using a rotablator. Burr selection should be incremental starting from the smallest sizes each time, followed by attempts at full stent expansion with non-compliant balloons. After complete stent expansion, additional DES implantation should be performed. Lifelong dual antiplatelet therapy should be considered in such patients. We should not forget the new proximal LAD lesion, which should be treated in the same session. If PCI of LAD is successful, a staged attempt for the RCA-CTO recanalisation should be considered, provided that the patient is still symptomatic and there is large corresponding reversible ischaemia.
Conflict of interest statement
The authors have no conflicts of interest to declare.
How would I treat?
THE INVITED EXPERTS’ OPINION
First of all, this case tells us how important lesion preparation is before stent implantation. If there is sign of a waist, which suggests incomplete dilation, we have to consider more aggressive lesion preparation with a non-compliant (NC) balloon, cutting balloon or rotablation before stent implantation.
If we were to encounter this situation after stent implantation, we would try to treat the lesion as follows.
The first step is a high-pressure (approximately 25 atm) balloon angioplasty with a short NC balloon (3×10 mm). Lesions with encircling calcification are much harder to dilate after stent implantation than before. A short balloon, 10 mm long, would be more appropriate to deliver the high pressure directly and effectively to the very waist of the lesion. The buddy wire technique might help to concentrate the inflation force during balloon dilatation like a cutting balloon6. Even if the waist of the lesion is not relieved with several attempts of high-pressure balloon angioplasty with a short NC balloon, repeating the angioplasty for more than 15 or 20 times may work due to continuous and effective delivery of inflation forces to the lesion.
The second step is a rotational atherectomy (RA) device. The RA device is the most popular mechanical debulking device7. The RA device is also used to ablate underexpanded stent struts as well as heavy calcification8. The RA procedure for an underexpanded stent usually and commonly creates greater friction forces9, which will impede burr rotation. In this case, the most important things to remember are to keep the rotational speed at more than 180,000 rpm during the whole RA procedure, and to advance the RA burr slowly and steadily. After RA with a 1.25 mm burr, high-pressure balloon dilatation with an NC balloon is performed. In particular, use of intravascular ultrasound after RA and balloon angioplasty would be highly recommended to evaluate the status of the underexpanded stent, underlying lesions including the severity of calcification and any potential acute complication during complex procedures (e.g., intramural haematoma or dissection).
Finally, coronary bypass surgery may be considered. The authors stated that they did not consider redo surgery, but the reasons were not clearly identified. The patient had a history of only single venous bypass graft to the right coronary artery, which suggests the presence of candidates for arterial grafts such as internal mammary arteries. If there are no contraindications for surgery, redo bypass graft surgery is worth considering, especially in that the right coronary artery graft was also occluded. If the risk of complications in the wound or other post-operational care is high, MIDCAB (minimally invasive direct coronary artery bypass) surgery for a bypass graft to the left anterior descending artery could be another option10, although it can also be of considerable risk or impossible because of adhesions and scars.
Conflict of interest statement
The authors have no conflicts of interest to declare.
How did I treat?
ACTUAL TREATMENT AND MANAGEMENT OF THE CASE
The decision of the Heart Team was to attempt at least one more percutaneous approach, a procedure to which the patient consented. The predefined sequential interventional strategy was as follows:
1) An 8 Fr extra support guiding catheter (for additional back-up and anticipated rotablator use).
2) Cutting balloon or similar technology.
3) Vessel wall and stent drilling by a 1.25 mm rotablator (stent ablation) burr in case of failure of the second step.
4) Use of a double-layer high-pressure balloon as the last option.
An 8 Fr Voda Left 3.5 guiding catheter (Boston Scientific, Natick, MA, USA) and a Balance Middleweight Universal II (BMW U II) guidewire (Abbott Vascular, Santa Clara, CA, USA) were used initially. A 2.5×10 mm Scoreflex (OrbusNeich, Hoevelaken, The Netherlands) deployed at 14 atmospheres (atm) failed to open the stent struts. Guidewire exchange over a FineCross™ MG (Terumo Corp., Tokyo, Japan) microcatheter was performed in order to perform rotablation. During the first run, the 1.25 mm burr stalled in the lesion, leading to drastic hypotension that immediately corrected upon gentle device retrieval. The burr crossed during the second run but the rotational speed was suboptimal and further rotablation was abandoned because of the anticipated risk of complications. Guidewire exchange was again performed over the microcatheter and a 3 m long Iron Man guidewire (Abbott Vascular, Santa Clara, CA, USA) was positioned. This support wire allowed positioning of a “bulky” 3.5×10 mm cutting balloon (Boston Scientific), inflated unsuccessfully at 18 atm at the lesion site (Figure 2). At this stage, crossing of the stent with an IVUS probe was unsuccessful.
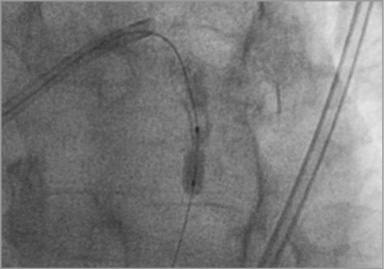
Figure 2. Inability to open the stent with a 3×10 mm cutting balloon at 18 atm.
Finally, “ultra-high-pressure balloon angioplasty” was chosen as a “last option” and an OPN NC® 3.0×10 mm balloon (SIS Medical AG, Winterthur, Switzerland) was progressively but slowly inflated. The maximal pressure on the stop manometer of the inflation device being 30 atm, pressure was further increased during a second round up to 42 atm. At this stage, the stent suddenly opened up (Figure 3). Of note, throughout the whole procedure the patient presented short episodes of severe but reversible hypotension during temporary vessel occlusion. This was accompanied by intense chest pain, requiring morphine.
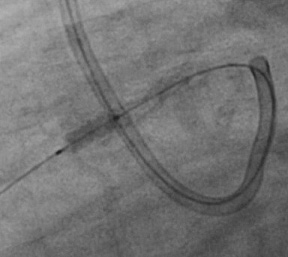
Figure 3. Opening of the stent at 42 atm by use of a 3×10 mm OPN NC balloon.
Angiography now demonstrated a widely patent vessel without perforation but potential stent fracture, a complication that was confirmed by IVUS (Figure 4). IVUS further demonstrated circumferential calcium that was particularly prominent from 11 to 2 o’clock and confirmed the presence of a significant proximal lesion (minimal cross-sectional area of 2.2 mm2). Therefore, the whole proximal segment, including the fracture site, was secured by implantation of a 3×18 mm and a 3.5×11 mm Biomatrix (Biosensors Europe, Morges, Switzerland) stent. Final angiography showed a good angiographic result (Figure 5A, Figure 5B). IVUS of the proximal LAD was performed and showed adequate apposition of the stents. The patient had an uneventful postprocedural clinical course and returned home the next day.
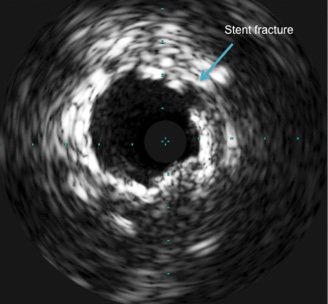
Figure 4. Stent fracture as demonstrated by IVUS.
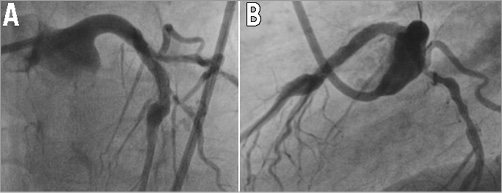
Figure 5. Coronary angiogram. A) Final result in the frontal view. B) Final result in the lateral view.
Discussion
Undeployed stents within “undilatable” lesions are a rare complication of PCI11-14. Even if one would expect this to happen more frequently because of the low crossing profile of current stents, progress in non-compliant balloon technology often enables this complication to be corrected. Underdeployment is typically partial and at the site of heavily calcified plaque. As stated previously, use of different non-compliant balloons up to burst pressure is most commonly performed. Balloon “explosion” and perforation is the most feared secondary complication but may potentially be anticipated by IVUS analysis of the true vessel size and calcium distribution. Anecdotal reports advocate the use of cutting balloon technology and rotablation (stent ablation). Unfortunately, these strategies, including IVUS analysis of the stented segment, failed in this particular patient. The inability to visualise the lesion site by IVUS prior to “ultra-high-pressure” angioplasty should be considered as a limitation of our approach. However, angiography demonstrated a reference diameter >3 mm proximal to the non-deployed stent segment and an aneurysmal LAD distal to it. Therefore, we anticipated the risk for perforation in this particular patient as being low.
The OPN NC balloon utilises a twin-layer balloon technology with highly resistant material. The instructions for use indicate a 35 atm burst pressure. However, in our limited experience we have not encountered balloon burst at pressures up to 45 atm. The only downside of the balloon is its bulky profile. In this patient, progressive balloon inflation was performed, which enabled opening up the stent at 42 atm without balloon explosion or vessel perforation. Stent fracture was corrected by additional stent placement resulting in an excellent angiographic and IVUS result.
In conclusion, ultra-high-pressure non-compliant balloon angioplasty may be performed successfully in selected cases to correct undeployed stents.
Conflict of interest statement
E. Eeckhout has research contracts with Abbott Vascular, Boston Scientific, Biosensors International, Stentys, Biotronik, and Medtronic, and is a proctor for St. Jude Medical. The other author has no conflicts of interest to declare.

