
Abstract
Since the first balloon angioplasty by Andreas Grüntzig 40 years ago, interventional cardiology has witnessed the introduction of countless tools and techniques that have significantly contributed to broadening the application of percutaneous coronary interventions (PCI) in unprecedented anatomic settings. Heavily calcified, fibrotic coronary stenosis has traditionally represented a very challenging scenario for PCI, and a very common indication for surgical revascularisation. This was mostly due to the difficulty in adequately dilating these lesions and/or to the inability to deliver and implant stents appropriately, which is often associated with high rates of procedural complications and suboptimal long-term clinical outcomes. Thanks to dedicated cutting and scoring balloons and to atherectomy devices, the treatment of most fibrotic and heavily calcified stenoses has become feasible and safe. Interventional cardiologists have learned how best to apply these tools through better patient and lesion selection, and also as a result of improved technology and techniques. In this review, we describe a 40-year-long journey that has evolved from the initial stand-alone debulking strategy to the currently applied coronary plaque modification, with the main objective of optimising drug-eluting stent delivery and implantation, translating into significantly improved patient outcomes.
Introduction
Andreas Grüntzig described the difficulty of treating calcified coronary stenoses in one of the initial papers on percutaneous coronary intervention (PCI)1. Forty years later, despite significant advances, heavily calcified lesions remain a challenge for successful PCI.
Advanced age, renal disease and diabetes have all been associated with coronary artery calcification (CAC), with severe CAC affecting between 6 and 20% of patients treated with PCI2. CAC is routinely encountered yet often underdiagnosed with angiography (Figure 1). Intravascular ultrasound (IVUS) studies have demonstrated that CAC is missed in nearly half of the cases with angiography alone3. Recognition of heavily calcified lesions allows appropriate utilisation of ablative techniques for initial vessel preparation.
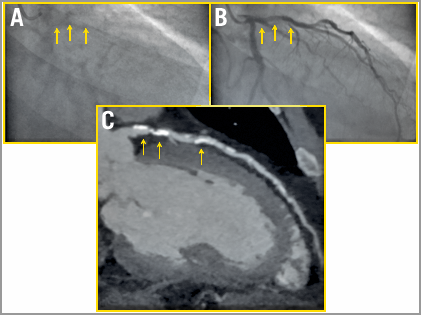
Figure 1. Patient with heavy coronary calcification underdiagnosed with angiography. Angiogram before (A) and during contrast injection (B) does not allow visualisation of the calcium burden of the proximal left anterior descending (LAD) artery (yellow arrows). The coronary computed tomography (C) reports an Agatston score of 839, with heavy calcification of the proximal LAD.
Heavily calcified lesions are difficult to dilate adequately and are associated with failure to deliver a stent, impaired drug delivery and possible polymer disruption with drug-eluting stents (DES), and stent underexpansion. Revascularisation of calcified lesions results in significantly increased periprocedural complications, long-term adverse events and high rates of restenosis2. Intravascular imaging has provided insights into the aetiology of in-stent restenosis (ISR) and the role that inadequate vessel preparation often plays4. To address the challenges faced by CAC, lesion preparation has evolved over the past four decades to help optimise the results of PCI (Figure 2).
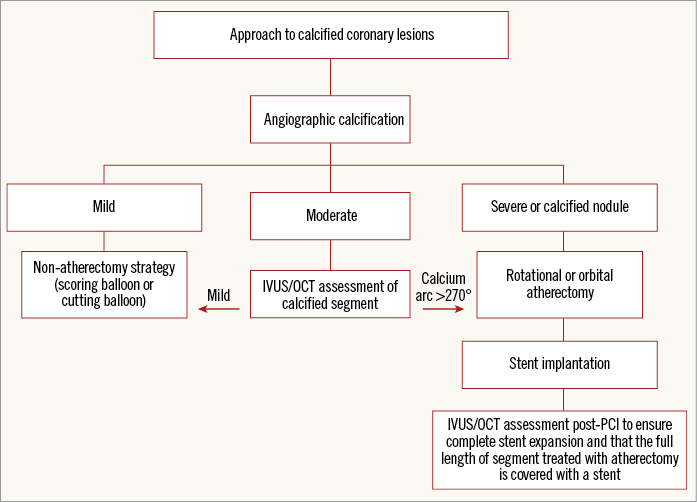
Figure 2. Optimal approach to calcified coronary lesions. IVUS: intravascular ultrasound; OCT: optical coherence tomography; PCI: percutaneous coronary intervention
Cutting and scoring balloons
With plain old balloon angioplasty (POBA), lesion dilatation occurs with forces directed in random directions across the vessel wall. Differential resistance to radial force within the lesion may result in multiple arcs of dissection. Additionally, when the arc of calcium/fibrosis extends to a large segment of the circumference of the vessel, the lesion may become resistant to high-pressure dilatation. Unsuccessful lesion expansion may also occur when the wall of the vessel opposite the arc of calcium is very compliant, thus allowing asymmetric balloon expansion, without fracturing the calcification. To address these clinical needs, modified balloon catheters have been developed.
Introduced in 1991, the Flextome™ Cutting Balloon™ Dilatation Device (Boston Scientific, Marlborough, MA, USA) is a 6, 10 or 15 mm-long balloon catheter with three (for 2.0-3.25 mm balloons) or four (on balloon sizes 3.5-4.0 mm) microblades (Figure 3). These blades, mounted longitudinally on the surface of a non-compliant balloon, are ~0.25 mm in height and five times sharper than conventional surgical blades5. During dilation, the device creates three or four endovascular radial incisions through the fibrocalcific tissue, thus allowing further expansion with conventional balloons. Additionally, the blades anchor into the intima, thereby preventing balloon slippage, something which is frequently seen in ISR.
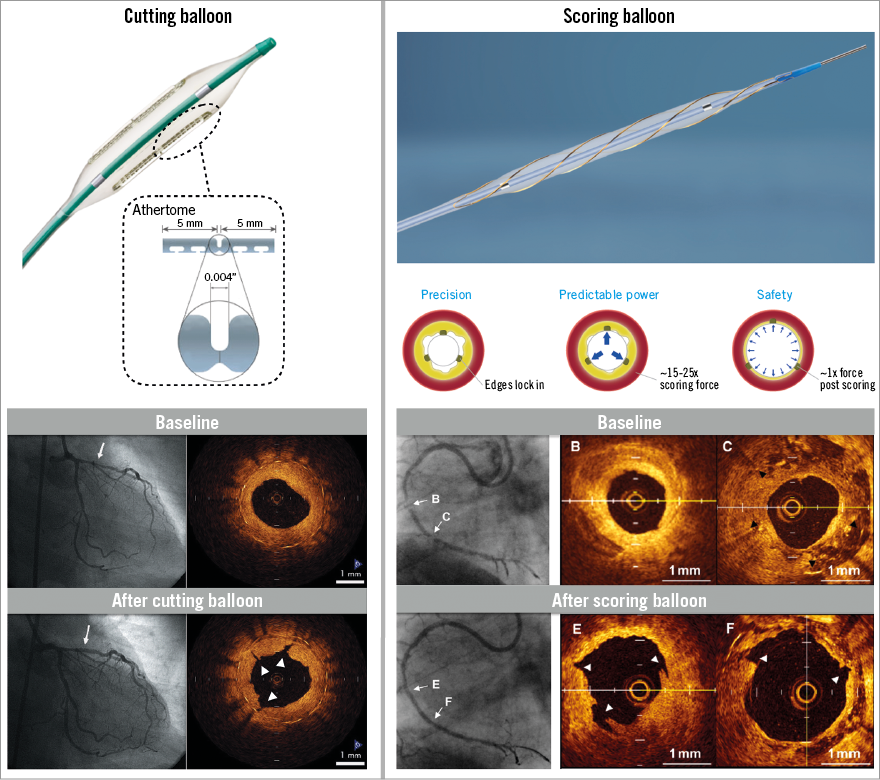
Figure 3. Case examples of coronary stenoses treated with cutting or scoring balloon. Left panel: Flextome cutting balloon. Bottom left: angiographic and OCT imaging of a case of ISR before and after treatment with cutting balloon. Three distinctive narrow fissures are observed (arrowheads), indicating plaque modification. Reproduced with permission from Kume et al40. Right panel: AngioSculpt scoring balloon. Bottom right: angiographic (A & D) and OCT imaging of a case of de novo lesion (B & E) and ISR (C & F) before and after treatment with scoring balloon. Fissures in both kinds of plaque can be observed (arrowheads in E and F). Reproduced with permission from Takano et al41.
By creating axial fissures, cutting balloon angioplasty (CBA) is more effective than POBA, and has been used to treat balloon-resistant lesions, such as de novo fibrocalcific plaques, as well as ISR. CBA achieves significantly larger luminal gain than POBA in patients with aorto-ostial lesions6. This has been linked with the effective fissures created in the muscular layers and elastic fibres around coronary ostia, which are otherwise prone to acute recoil following conventional angioplasty. An IVUS-based study7 indicated that CBA achieves larger luminal gain than POBA in calcified lesions. Another appealing angiographic scenario is represented by small-vessel disease, which is particularly prone to acute dissection and restenosis after POBA. In the CAPAS trial8, CBA was associated with a lower rate of binary restenosis than POBA at three months. The Cutting Balloon Global Randomized Trial was the largest randomised trial (n=1,238) comparing CBA with POBA for the prevention of restenosis in de novo lesions9. Acute procedural success was similar between groups. The rate of perforation was higher with CBA (0.8% vs. 0%, p=0.03). The primary endpoint, six-month binary restenosis, did not differ between CBA and POBA (31% vs. 30%, p=0.75).
These negative results, together with the higher rate of perforation and difficulties associated with cutting balloon delivery (due to its high crossing profile: 0.041”-0.046”), led to the development of alternative balloon-based atherectomy devices, such as scoring balloons. AngioSculpt® (Spectranetics, Colorado Springs, CO, USA) consists of a semi-compliant nylon balloon, surrounded by three external nitinol spiral scoring wires (Figure 2). This device is more flexible and deliverable than the cutting balloon (the crossing profile of the smallest device is 0.036”), and is available in lengths of 10, 15, and 20 mm, and diameters of 2.0-3.5 mm. In the feasibility trial10, AngioSculpt with routine bare metal stent (BMS) implantation was used for de novo lesions, and AngioSculpt alone was evaluated for the treatment of BMS restenosis. Procedural success was 100%. Two type A dissections following AngioSculpt, and no perforations were reported. The target lesion revascularisation (TLR) rate was 10% at six months. Another trial (personal communication) included 200 patients with calcified lesions or ISR. Procedural success was 98%. Dissections occurred in 14%. There were no device-related perforations. The major adverse cardiovascular events (MACE) rate at 21 days was 2.5%. Grenadier et al treated 521 patients (75% with calcified lesions) (personal communication). Procedural success was 98%, while the 30-day MACE rate was 3%. At a median of 34 months, the MACE rate was 7% (TLR 6%). In an observational study11 including 299 patients undergoing IVUS-guided coronary DES implantation, the AngioSculpt enhanced stent expansion, compared with direct stenting and POBA with semi-compliant balloons. Finally, Miyazaki et al12 reported on 184 lesions treated with POBA vs. AngioSculpt prior to bioresorbable scaffold (BRS) implantation. Despite the utilisation of AngioSculpt in a higher proportion of type B2/C, restenotic, and calcified lesions, the scoring balloon group demonstrated better procedural IVUS outcomes with regard to both scaffold expansion and eccentricity. The one-year TLR rate was similar between groups (conventional balloon 6% vs. AngioSculpt 7%; p=0.87).
Unfortunately, no randomised comparison exists between cutting and scoring balloons, and any consideration on the comparative performance of these devices is speculative. In our practice, the cutting balloon is preferentially used to treat ISR due to the stability of the device inside the lesion and the possibility to reach >20 atm (low compliance). However, scoring balloons can also be used very effectively to treat ISR, taking into account the higher compliance of these devices. Scoring balloons are preferentially utilised in preparing de novo lesions. In our practice, we tend to undersize the scoring balloon, inflating it at 20-22 atm.
Atherectomy
A strategy for debulking calcified lesions as part of a bail-out technique to address undilatable stenoses has evolved into an approach of lesion preparation by plaque modification. Primary atherectomy is associated with decreased procedural and fluoroscopy times, contrast volume, and the number of predilation balloon catheters used when compared to bail-out atherectomy13. Lesion preparation with atherectomy has emerged as a strategy that alters plaque morphology, creating fractures in the calcified lesion and changing lesion compliance, to increase the likelihood of maximal luminal gain and complete stent expansion. Atherectomy is currently achieved with either rotational or orbital atherectomy.
Rotational atherectomy
Percutaneous transluminal rotational atherectomy (PTRA) was introduced in 1988 by David Auth, primarily to achieve mechanical debulking of atherosclerotic plaque. Differential cutting induced by PTRA allows mechanical ablation of inelastic fibrocalcific plaques while sparing adjacent elastic tissue that deflects away from the ablating burr (Figure 4). PTRA results in plaque modification with dissections occurring less frequently as compared to balloon angioplasty. The first-in-human study by Fourier validated the safety and effectiveness of PTRA14. The initial enthusiasm was sobered by the high rate of TLR observed with PTRA versus balloon angioplasty (42 vs. 32%, p=0.013)15. The same results were reproduced by subsequent studies highlighting the limitations of PTRA as a “stand-alone” revascularisation strategy16,17.
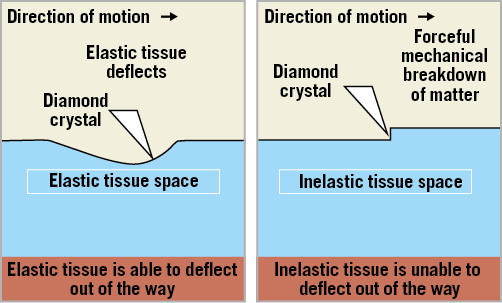
Figure 4. High-speed rotational ablation differentiates healthy elastic vessel wall from inelastic plaque.
Several modifications in the primary technique have been introduced to reduce the restenosis rate. The STRATAS and CARAT trials randomised 719 patients to a less or more aggressive debulking strategy based on the burr to artery ratio lower or higher than 0.7, respectively18,19. The acute angiographic results and six-month clinical endpoints were not different between the two strategies, while an excess of procedural complications (e.g., perforation, no-reflow, large dissection) was reported with the more aggressive debulking strategy. In addition, the SARS study investigated the impact of reducing the burr speed during rotablation to 140-160 K revolutions per minute (rpm), while at the same time avoiding deceleration >3,000 rpm20. The latest was achieved by introducing the so-called “pecking motion”, a forward-backward movement of the burr in order to reduce the effective ablation time and contact of the burr with the plaque. These modifications were associated with less platelet activation21, and significant reductions in procedural complications, though no difference in restenosis rate was seen.
The advent of BMS conceived to reduce the restenosis rate after POBA prompted a novel application of rotational atherectomy22 (Theodore M. Bass, MD, Shands Jacksonville Hospital, Jacksonville, Florida, unpublished data, February 2003). PTRA was thought to be a useful technique to prepare the vessel and modify the plaque better, facilitating the delivery and full expansion of stents.
CONTEMPORARY EVIDENCE
In recent years, PTRA has been increasingly adopted following the excellent performance of DES. Studies have shown decreased ISR and TLR with PTRA followed by DES as compared with PTRA followed by BMS23,24.
The need for PTRA before DES implantation stems from a 6% failure rate to deliver and a twofold higher failure rate to deploy DES successfully in heavily calcified stenoses25. PTRA might overcome these potential limitations by obtaining adequate plaque modification and lesion preparation before DES implantation. In fact, contemporary observational studies and registries have consistently demonstrated favourable results of DES implantation in heavily calcified lesions after PTRA (Table 1). Yet, a randomised clinical trial failed to demonstrate a superiority of PTRA versus conventional balloon dilatation before DES implantation in the treatment of heavily calcified coronary stenoses26. This highlights the limitations of including these complex patients in randomised trials. The patients with heavily calcified coronary stenoses simply cannot be treated percutaneously without PTRA, and therefore cannot be randomised, as confirmed by the 8% higher crossover rate from balloon dilatation to PTRA observed in the ROTAXUS trial26.
The latest guidelines reflected the available data, granting a Class IIa (level of evidence C) to PTRA, recommended for the preparation of heavily calcified or severely fibrotic lesions that cannot be crossed by a balloon or adequately dilated before planned stenting27.
THE ROTABLATOR SYSTEM
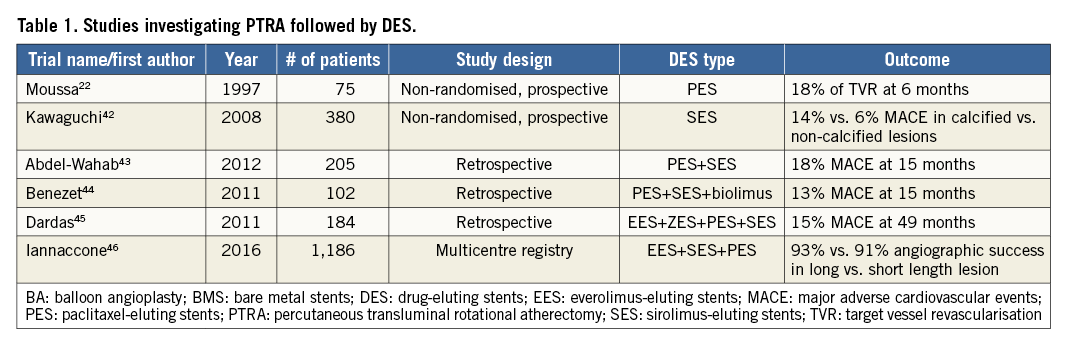
The Rotablator® system (Boston Scientific) is composed mainly of the advancer, the console and the burr.
The advancer has a burr knob control on the upper part, and on the side it has a saline infusion port, a fibre optic tachometer cable connection, and an advancer hose with the compressed gas connector (Figure 5). The burr control knob allows sliding the knob forward or backward to advance or retract the burr to its starting position. The connection to the cylinder of air-nitrogen gas enables the turbine within the advancer to rotate the driveshaft. The saline infusion port allows reducing heat, friction, and spasm. The foot pedal activates the burr, while the small knob next to it allows switching from rotablator mode to Dynaglide™ mode. The Dynaglide mode enables the burr to rotate at a fixed speed between 60 and 90 K rpm to facilitate burr exchange.
Figure 5. Rotablator system.
The nickel-plated burr has an elliptical shape and is coated with 2-3,000 microscopic (20 to 30 mm) diamond crystals. The burr is attached to a 135 cm-long nickel-plated driveshaft, covered by a Teflon sheath that allows the saline solution to cool and lubricate the rotating components while preventing possible driveshaft-induced injury to the artery.
The Rotablator system is an over-the-wire device, with the guidewire entering the proximal end of the burr and exiting the back end of the advancer body. Common 0.014-inch guidewires are not compatible with the Rotablator system. Two dedicated thin rotawires (0.009-inch) are available: the floppy and extra support versions. A more detailed description of the tools and practical set-up of the Rotablator system can be found in the EAPCI-PCR Textbook and on PCR edu online28,29.
CONTEMPORARY PTRA TECHNIQUE
Contemporary PTRA technique reflects the long-standing experience and the large body of evidence accumulated over the years eventually supporting the paradigm shift from the initial aggressive stand-alone debulking strategy to plaque modification mainly aiming at optimal DES implantation. This has been the object of a recent consensus document generated by a group of highly experienced operators30. Several aspects of PTRA were refined, from the procedural set-up to the tools and techniques applied. The arterial access that was traditionally a large 8 Fr femoral sheath is now a contemporary 6 Fr radial or femoral sheath. The wiring technique has been facilitated by the use of a regular workhorse wire or hydrophilic wire subsequently exchanged over microcatheters or over-the-wire balloons with the rotawire. The currently recommended burr is a single 1.5 mm burr to tackle different lesion characteristics, achieving good plaque modification, i.e., a burr-to-artery ratio of 0.6, while respecting budget constraints. The ablation speed has been lowered as compared to the initial protocol and aims at a range of 135 to 180 K rpm. Much emphasis is given to the deceleration speed that should not be higher than 5,000 rpm to reduce possible complications (e.g., burr lodging). The need for a temporary pacemaker has been reconsidered by many experienced operators who report a lower incidence of transient heart block with smaller burrs and lower speeds, easily manageable with the use of atropine. With adequate training and experience, PTRA can be a very safe technique (Figure 6).
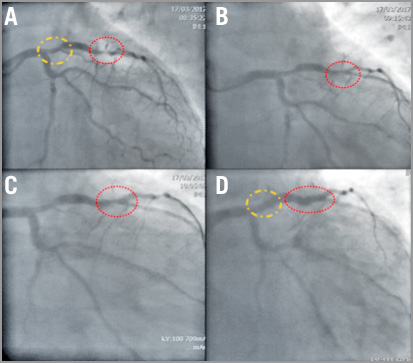
Figure 6. Case example of a heavily calcified LAD stenosis treated with provisional PTRA after failed attempt with NC balloon. A) 50% stenosis of the proximal LAD (yellow circle) and severely calcified stenosis of the mid LAD (red circle). B) Suboptimal result after three consecutive dilatations with non-compliant balloons of increasing size (from 2.5 to 3.25 mm). C) Angiographic result after rotablation with 1.5 mm burr. D) Final angiographic result after stent deployment in both proximal and mid LAD.
Orbital atherectomy
The Diamondback 360° Coronary Orbital Atherectomy System (OAS) (Cardiovascular Systems Inc., St. Paul, MN, USA) is a percutaneous device indicated to facilitate stent delivery in patients with coronary artery disease (CAD) who are candidates for stenting due to de novo, severely calcified CAD. Orbital atherectomy (OA) utilises an eccentrically mounted 1.25 mm diamond-coated crown on a 6 Fr compatible ViperWire™ guidewire (Cardiovascular Systems Inc.) with an electric, hand-controlled console to control crown movements (Figure 7). A proprietary lubricant, ViperSlide® (Cardiovascular Systems Inc.), flushes the system, reducing friction while the crown ablates calcium at low or high speeds (80 or 120 K rpm, respectively). The orbiting crown has a unique mechanism of action that incorporates differential sanding using centrifugal force to ablate hard, non-compliant calcium while flexing away from normal, healthy tissue leaving it intact. Advancing the OA travel knob slowly can increase the radius of orbit, creating fractures within the calcium31.
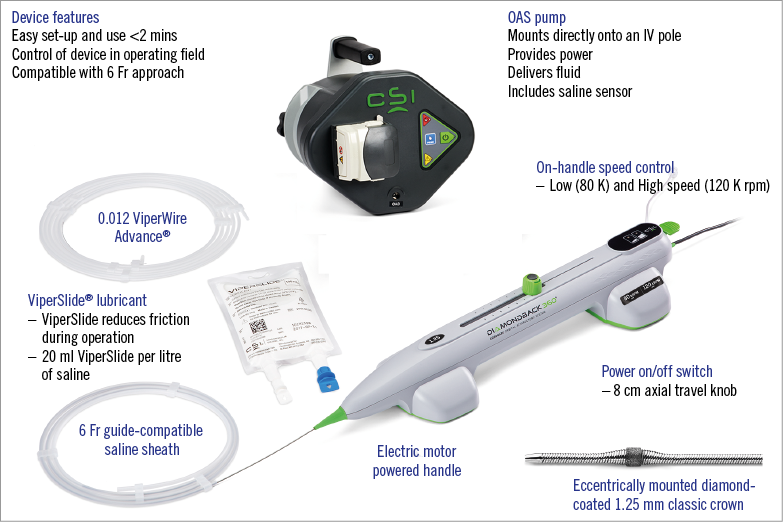
Figure 7. Diamondback 360° Coronary Orbital Atherectomy System. Used with permission from Cardiovascular Systems, Inc.
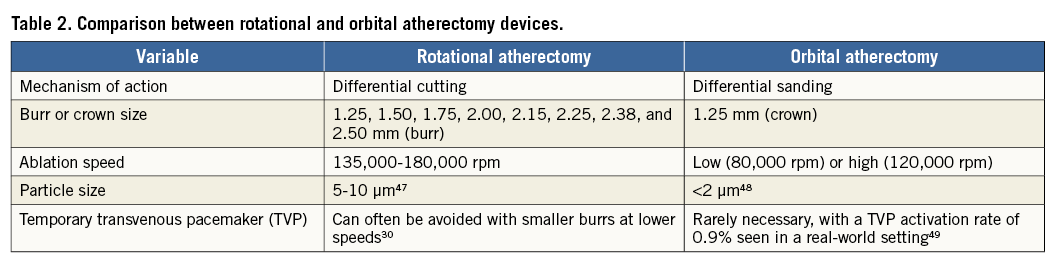
Coronary OA has a number of technical aspects that distinguish it from PTRA (Table 2). In addition to advantages including a more rapid set-up with use of a single size burr, bi-directional ablation might reduce the likelihood of crown entrapment. Production of microparticulate debris averaging less than 2 mm in size with continuous blood flow maintained during ablation helps to reduce thermal injury, transient heartblock and the no-reflow phenomenon31. OA was found to result in deeper tissue modification as compared to PTRA by optical coherence tomography (OCT) assessment32. This may lead to improved stent strut apposition and more complete stent expansion following lesion preparation with OA, though no large-scale multicentre randomised head-to-head trials have yet occurred comparing the two devices.
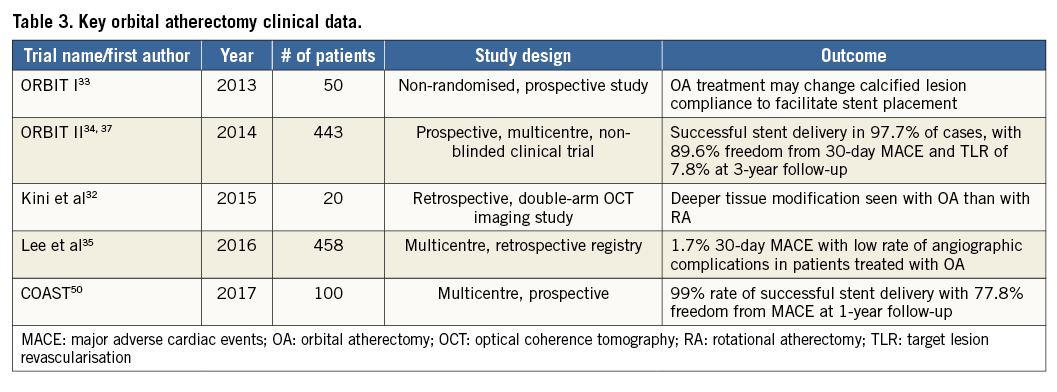
Orbital atherectomy was approved in the USA in 2013, for lesion preparation of severely calcified coronary lesions prior to stent implantation. Commercial use of OA is currently limited to the USA and Japan. Data on clinical outcomes of OA are largely based on single-arm studies including the ORBIT I trial (Safety and feasibility of orbital atherectomy for the treatment of calcified coronary lesions), ORBIT II trial (Evaluate the Safety and Efficacy of OAS in Treating Severely Calcified Coronary Lesions), and a real-world multicentre registry (Lee et al) that demonstrated safety and efficacy of OA with low rates of angiographic complications and MACE33-36. The durable benefits of OA were demonstrated in the ORBIT II trial, with a three-year TLR rate of 7.8% in patients with severe CAC37 (Table 3).
To supplement existing data, the Evaluation of Treatment Strategies for Severe CaLcifIc Coronary Arteries: Orbital Atherectomy vs. Conventional Angioplasty Prior to Implantation of Drug Eluting StEnts (ECLIPSE trial) was designed to assess the role of OA. The ECLIPSE trial is enrolling approximately 2,000 patients to be treated with either OA vessel preparation or conventional angioplasty, in what will be the largest randomised trial to evaluate patients with severe CAC (clinicaltrials.gov: NCT03108456). An additional OA device has been developed that includes a micro crown system incorporating a diamond-coated tip in addition to the classic crown, and was evaluated in patients in the Coronary Orbital Atherectomy System Study (COAST) trial (personal communication).
Excimer laser coronary atherectomy
Excimer laser coronary atherectomy (ELCA), by means of a photoacoustic mechanism, has been used to treat resistant coronary stenoses. In analogy to PTRA, several modifications to ELCA have been introduced to reduce the procedural complications and high restenosis rate38, such that it could be an option in the treatment of complex coronary lesions with moderate calcification before DES implantation39.
Authors’ perspectives and future directions
Improvements in balloon catheter flexibility and crossing profile, as well as the addition of hydrophilic coatings, will improve the deliverability (and hence the utilisation) of cutting and scoring balloons. Moreover, the addition of an antiproliferative drug release system would streamline the use of such devices in small-vessel disease and ISR, thus combining the benefit of balloon-based atherectomy and the antiproliferative properties of drug-eluting balloons. Further refinements of the cutting/scoring balloon concept have also been undertaken. For example, the Chocolate® balloon (QT Vascular Ltd, Singapore) features a mounted nitinol constraining structure (“pillows-and-grooves design”) specifically designed for uniform, focused, controlled inflation and rapid deflation, resulting in atraumatic dilatation with minimal risk for dissection. Currently available evidence is limited to case reports; prospective studies are needed to evaluate its efficacy and safety in large cohorts.
A new generation of Rotablator system is being launched with major modifications mainly directed to simplifying its use. The foot pedal will be eliminated and replaced by a button positioned on the top of the burr control knob of the advancer. Another button is foreseen on the side of the advancer to replace the small knob of the pedal used to switch to Dynaglide mode. The console will be smaller, requiring less set-up time, available either with horizontal or vertical display enabling mounting on the IV pole of the cathlab table.
The Shockwave Medical Coronary Rx Lithoplasty System (Shockwave Medical Inc., Fremont, CA, USA) is a novel balloon catheter-based device that utilises pulsatile mechanical energy to disrupt calcified lesions using technology similar to lithotripsy for kidney stones. The Conformité Européenne (CE) mark was granted in May 2017, based on initial results of the DISRUPT CAD I pre-market, prospective, multicentre, single-arm study that was designed to evaluate the safety and efficacy of lithoplasty in 60 patients (personal communication).
Conflict of interest statement
E. Barbato declares consultancy and speaker’s fees on his behalf to the Cardiovascular Research Institute Aalst from Boston Scientific. E. Shlofmitz has received honoraria from Cardiovascular Systems, Inc. R. Shlofmitz is a consultant for Cardiovascular Systems, Inc., and Abbott. L. Azzalini has received honoraria from Guerbet and research support from ACIST Medical Systems. The other authors have no conflicts of interest to declare.

