Introduction
In the randomised High-Speed Rotational Atherectomy Versus Modified Balloons Prior to Drug-Eluting Stent Implantation in Severely Calcified Coronary Lesions (PREPARE-CALC) trial, lesion preparation using rotational atherectomy (RA) prior to sirolimus-eluting stent (SES) implantation improved procedural success compared to modified balloons (MB; cutting or scoring). On the other hand, acute lumen gain and stent expansion were similar in both treatment arms and RA was not associated with excessive late lumen loss at 9 months12.
The comparison of long-term clinical outcomes in patients with calcified lesions treated with RA versus MB followed by the implantation of new-generation drug-eluting stents (DES) has so far not been addressed in a randomised setting. The present analysis reports the 2-year clinical results of the randomised PREPARE-CALC trial.
Methods
The study design, the results of the primary endpoints and the 9-month clinical outcome have been published previously1. Briefly, PREPARE-CALC is a prospective, randomised trial in which 200 patients with complex calcified coronary lesions and documented ischaemia were randomly assigned 1:1 to either RA or MB followed by SES (Orsiro; Biotronik) implantation.
The principal endpoint of this analysis was target vessel failure (TVF) at 2-year clinical follow-up, defined as the composite of cardiac death, target vessel-related myocardial infarction (MI) or clinically driven target vessel revascularisation (TVR). Major adverse cardiac events (MACE) were defined as a composite of all-cause death, MI or TVR.
Results
Clinical follow-up at two years was completed in 97% of the study population. Overall, the cumulative incidence of TVF was 11% in the MB group versus 9% in the RA group (hazard ratio [HR] 1.23, 95% confidence interval [CI]: 0.51-2.96; p=0.648) (Figure 1A). The rates of all-cause death (8% vs 7%, HR 1.13, 95% CI: 0.41-3.31; p=0.807), TVR (9% vs 5%, HR 1.85, 95% CI: 0.62-5.51; p=0.265) and MI (5% vs 4%, HR 1.25, 95% CI: 0.34-4.67; p=0.735) did not differ significantly between both groups, resulting in similar MACE rates (18% vs 16%, HR 1.13, 95% CI: 0.58-2.22; p=0.722) (Figure 1B). Of note, a trend towards a higher rate of TLR in the MB group was observed (8% vs 2%, HR 4.1, 95% CI: 0.87-36.38; p=0.053) (Figure 1C, Supplementary Table 1).
With regard to TVF, results were consistent with the overall cohort in the majority of the post hoc subgroups (Supplementary Figure 1). We observed a significant interaction between treatment strategy and lesion length (p=0.045) favouring RA in the subgroup of patients with long lesions (≥27 mm; 19% vs 6.3%, HR 2.74, 95% CI: 0.82-9.09; p=0.10) and MB in patients with short lesions (<27 mm; 5.2% vs 11.1%, HR 0.46, 95% CI: 0.11-1.91; p=0.283).
After multivariable adjustment, only diabetes mellitus was associated with the occurrence of TVF at follow-up (HR 3.67, 95% CI: 1.04-12.85; p=0.043) (Supplementary Table 2). Ostial location (HR 11.3, 95% CI: 2.26-56.8; p=0.003) and lesion length (HR 1.06, 95% CI: 1.02-1.09; p=0.003) were independently associated with the time-dependent occurrence of TLR (Supplementary Table 3).
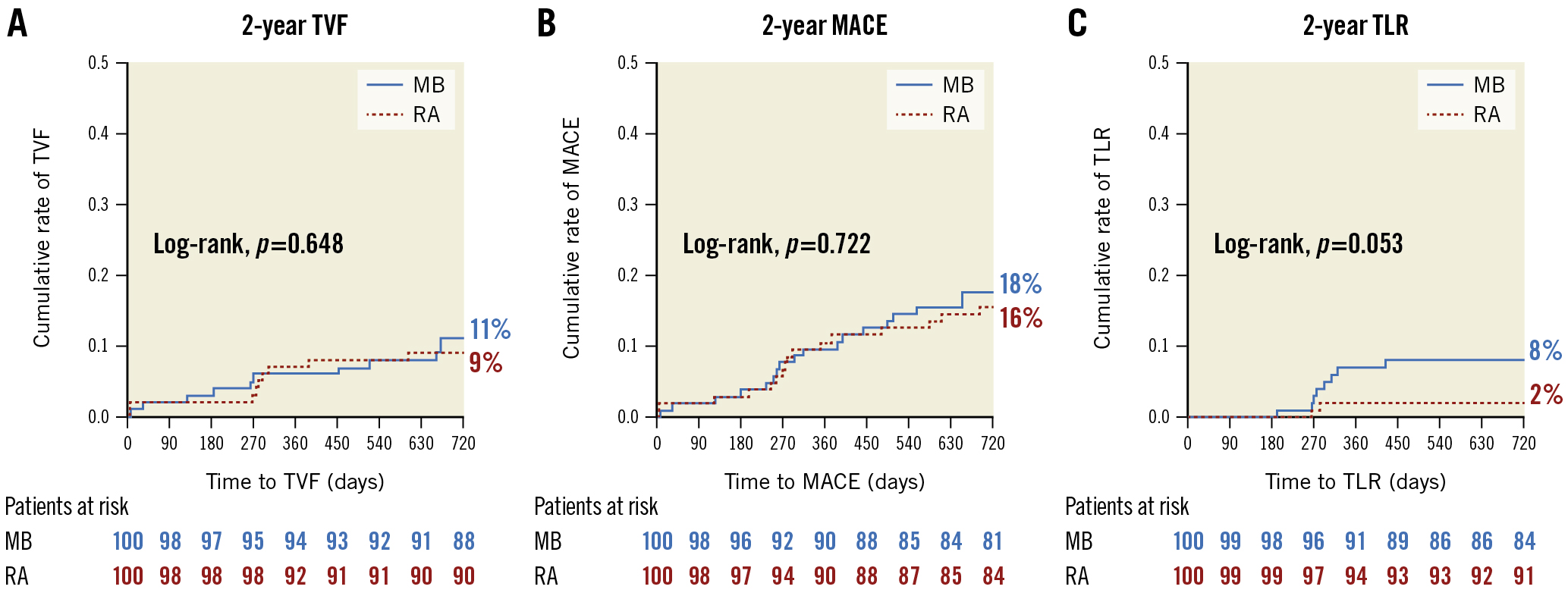
Figure 1. Clinical outcome at 2 years. Kaplan-Meier curves for the cumulative incidence of (A) TVF, (B) MACE and (C) TLR at 2 years in patients undergoing lesion preparation using rotational atherectomy versus modified balloons. MACE: major adverse cardiac events; MB: modified balloon; RA: rotational atherectomy; TLR: target lesion revascularisation; TVF: target vessel failure
Discussion
After encouraging in-hospital and 9-month follow-up data1, the current analysis confirms excellent results with both the MB and RA strategies at 2-year follow-up. These findings could be related to several factors, including the use of new-generation DES3, intravascular imaging guidance, as well as significant operator and centre experience in the treatment of severely calcified coronary lesions.
The rates of TVF and MACE at 2 years were comparable in both treatment arms. It is noteworthy that, in addition to the well-balanced baseline clinical and angiographic characteristics, lesion morphology on optical coherence tomography (OCT) analysis was similar in both arms2. In addition, after treatment, acute lumen gain and 9-month late lumen loss as well as stent results as assessed by OCT did not differ significantly between both treatment strategies12. This finding underlines the importance of proper lesion preparation and optimal acute stent results regardless of which technique is used to maintain favourable long-term outcomes.
The current analysis suggests that RA may be particularly favourable for longer calcified lesions, whereas MB may be especially suitable for shorter ones. This result is in line with previous reports showing the efficacy of atheroablative techniques in long lesions45, and might be partially explained by the ability of atheroablation to prepare uncrossable long lesions, overcoming the crossability issue of MB. Notwithstanding this, further studies are needed to explore the role of different lesion preparation techniques in long versus short lesions.
Limitations
First, scoring (rather than cutting) balloons were used in most cases in the MB group. Second, acute coronary syndromes were excluded, and therefore, findings only apply to patients presenting with chronic coronary syndromes. Finally, the sample size may be too small to draw definitive conclusions concerning clinical endpoints.
Conclusions
In patients with complex calcified coronary lesions, the rates of TVF and MACE at 2 years were not significantly different after lesion preparation with MB versus RA followed by newer-generation SES implantation. Compared to older reports, this study shows excellent long-term results and low revascularisation rates with both treatment forms. RA might be more effective in reducing TVF in long lesions, whereas MB appear to be more effective in short ones.
Funding
This study is funded by the Heart Center, Segeberger Kliniken GmbH, Bad Segeberg, Germany.
Conflict of interest statement
A. Allali is a consultant and proctor for Boston Scientific. G. Richardt has received institutional research grants from St. Jude Medical, Biotronik and Medtronic. T. Rheude received lecture fees from SIS-Medical AG. M. Abdel-Wahab reports that his hospital receives speaker honoraria and/or consultancy fees on his behalf from Boston Scientific and Medtronic. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

