Abstract
BACKGROUND: Percutaneous coronary intervention (PCI) of calcified lesions remains challenging for interventionalists.
AIMS: We aimed to investigate whether combining rotational atherectomy (RA) with cutting balloon angioplasty (RA+CBA) results in more optimal stent expansion compared with RA followed by non-compliant balloon angioplasty (RA+NCBA).
METHODS: ROTA-CUT is a prospective, multicentre, randomised trial of 60 patients with coronary artery disease undergoing PCI of moderately or severely calcified lesions with drug-eluting stent implantation. Patients were randomised 1:1 to either RA+CBA or RA+NCBA. The primary endpoint was the minimum stent area on intravascular ultrasound (IVUS). Secondary endpoints included minimum lumen area and stent expansion assessed by IVUS and acute lumen gain, final residual diameter stenosis and minimum lumen diameter assessed by angiography. Clinical endpoints were obtained at 30 days.
RESULTS: The mean age was 71.1±9.4 years, and 22% were women. The procedural details of RA were similar between groups, as were procedure duration and contrast use. Minimum stent area was similar with RA+CBA versus RA+NCBA (6.7±1.7 mm2 vs 6.9±1.8 mm2; p=0.685). Furthermore, there were no significant differences regarding the other IVUS and angiographic endpoints. Procedural complications were rare, and 30-day clinical events included 2 myocardial infarctions and 1 target vessel revascularisation in the RA+CBA group and 1 myocardial infarction in the RA+NCBA group.
CONCLUSIONS: Combining RA with CBA resulted in a similar minimum stent area compared with RA followed by NCBA in patients undergoing PCI of moderately or severely calcified lesions. RA followed by CBA was safe with rare procedural complications and few clinical adverse events at 30 days.
Moderate to severe coronary calcification in patients undergoing percutaneous coronary intervention (PCI) is common, with some studies reporting a prevalence of >30%12. This high prevalence is likely to increase further, considering the ageing of the population and the steady increase of comorbidities such as diabetes and chronic kidney disease34. Patients undergoing PCI of calcified lesions experience a high rate of major adverse cardiovascular events (MACE), including in the contemporary drug-eluting stent era56. Rotational atherectomy (RA) is an established tool to facilitate PCI of calcified coronary lesions7. More specifically, RA can modify physical attributes of calcified plaque and, thereby, facilitate balloon angioplasty and stent deployment. Despite higher strategic success with RA versus without RA, rates of restenosis and MACE have remained high after PCI of calcified lesions, regardless of whether RA was used8. Combining RA with other types of lesion preparation may improve acute procedural outcomes and, thus, longer-term outcomes. We designed the ROTA-CUT trial based on the hypothesis that a strategy of calcified lesion preparation with RA followed by cutting balloon angioplasty (RA+CBA) compared with RA followed by non-compliant balloon angioplasty (RA+NCBA) will result in more optimal stent expansion.
Methods
TRIAL DESIGN
The ROTA-CUT trial (U.S. Food and Drug Administration Investigational Device Exemption [FDA IDE] #G210030; ClinicalTrials.gov: NCT04865588) is a prospective randomised study of 60 patients undergoing PCI with drug-eluting stent implantation for moderately or severely calcified lesions. The study was conducted at Mount Sinai Hospital, New York, NY, USA and St. Francis Hospital & Heart Center, Roslyn, NY, USA. The Icahn School of Medicine at Mount Sinai designed and sponsored the trial, supported by an investigator-initiated grant from Boston Scientific. The institutional review boards of the two participating centres approved the trial protocol. Follow-up for clinical endpoints was obtained by telephone at 30 days and is ongoing for 270 days.
STUDY POPULATION
Patients undergoing PCI for a de novo calcified lesion with planned RA and planned drug-eluting stent implantation of a lesion with a target vessel reference diameter ≥2.5 mm and ≤4.0 mm, a lesion length ≥5 mm and moderate or severe calcification by angiography were eligible for enrolment in the trial. Severe calcification was defined as the presence of radiopacities noted without cardiac motion prior to contrast injection involving both sides of the arterial wall in at least one location and a total length of calcium (including segmented) of at least 15 mm extending partially into the target lesion area. Moderate calcium was defined as the presence of radiopacities only during the cardiac cycle before contrast injection with calcium extending partially into the target lesion69. Clinical exclusion criteria included cardiogenic shock, PCI for ST-segment elevation myocardial infarction, planned surgery within 6 months after the index PCI (unless dual antiplatelet therapy can be maintained throughout the perisurgical period), life expectancy less than 12 months and referral to coronary artery bypass grafting after a Heart Team discussion. Angiographic exclusion criteria included lesions with angulation >45 degrees by visual estimate, lesion stenoses through which a guidewire will not pass, lesions in a last remaining vessel with a left ventricular ejection fraction <30%, lesions in saphenous vein grafts, angiographic evidence of thrombus or significant dissection at the treatment site, and lesions within 10 mm of a previously placed stent. A full list of inclusion and exclusion criteria is provided in Supplementary Table 1.
STUDY TREATMENT
Patients who met all inclusion and no exclusion criteria and who had signed an informed consent before proceeding to the catheterisation laboratory were randomised before the planned use of RA but after the guidewire had successfully passed the lesion. Randomisation was an integrated functionality of the electronic data capture system and was conducted on a 1:1 basis to either RA+CBA or RA+NCBA (Central illustration). RA with the ROTAPRO (Boston Scientific) device was performed using a maximum burr-to-artery ratio of 0.4-0.6, a burr speed of 140,000-160,000 rotations per minute (rpm), and a maximum run duration of 20 seconds. Burr upsizing during the procedure was allowed, but the maximum burr-to-artery ratio was not to exceed the recommended ratio of 0.6. In patients randomised to RA+CBA, cutting balloon atherotomy using a WOLVERINE cutting balloon (Boston Scientific) was performed after RA. The WOLVERINE cutting balloon had to be sized 1:1 relative to the reference vessel diameter, and the recommended inflation pressure was 6 atm and was expected not to exceed the device-rated burst pressure of 12 atm. The choice of drug-eluting stent and the use of post-dilatation were at the operator’s discretion.
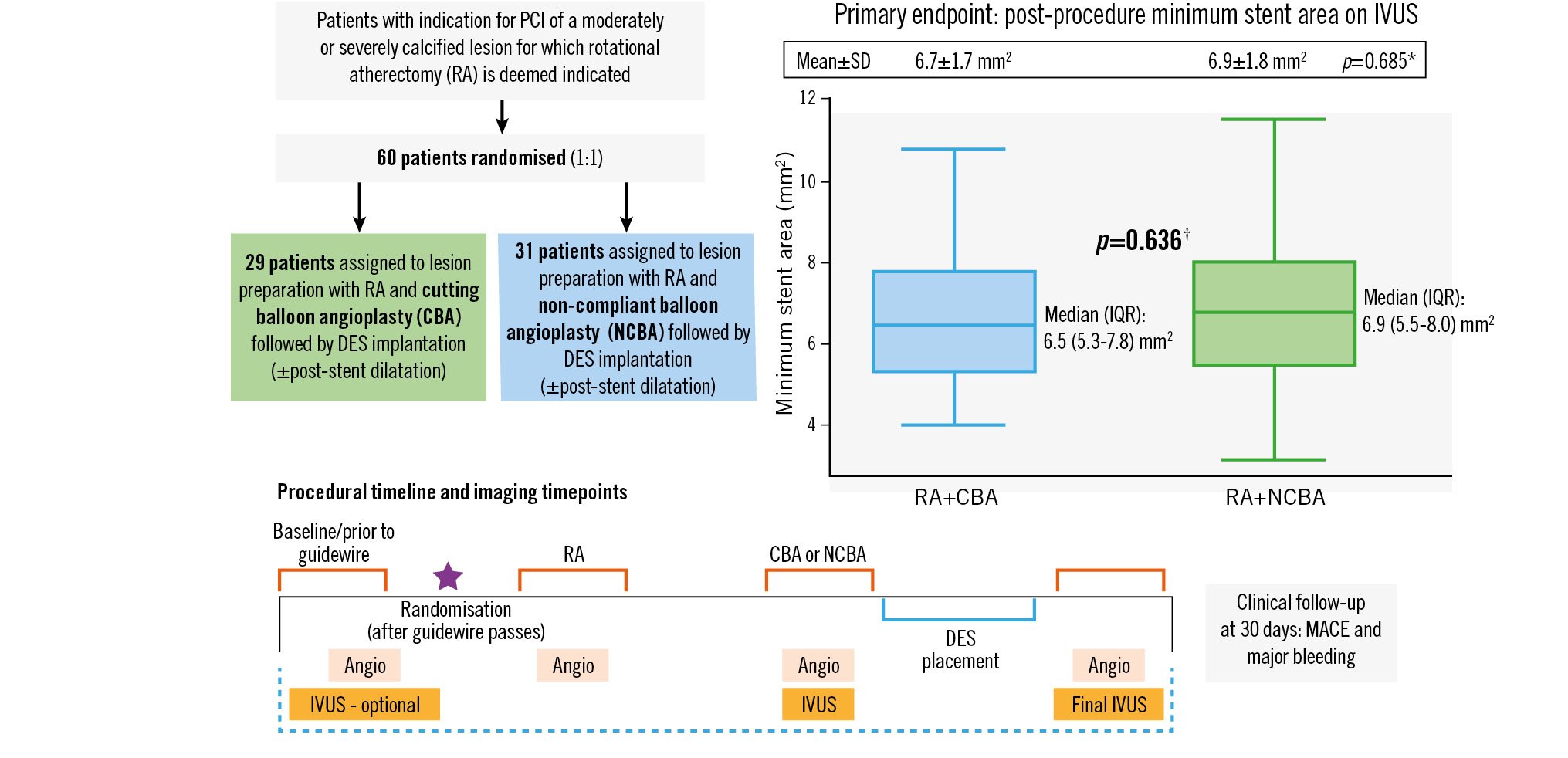
Central illustration. Design and primary outcome of the ROTA-CUT trial. A total of 60 patients were randomised to either rotational atherectomy (RA) followed by cutting balloon angioplasty (RA+CBA) or RA followed by non-compliant balloon angioplasty (RA+NCBA). Coronary angiography for offline quantitative coronary angiography (QCA) was performed at baseline, after RA, after RA+CBA/RA+NCBA, and at the end of the procedure. Intravascular ultrasound (IVUS) runs were performed at baseline (if possible), after lesion preparation defined as RA+CBA or RA+NCBA, and at the end of the procedure. The primary endpoint of the study was minimum stent area (in mm2) on IVUS. Clinical endpoints were collected at 30 days. *p-value for comparison of means±SD. † p-value for comparison of medians (IQR). Angio: angiography; DES: drug-eluting stent; IQR: interquartile range; MACE: major adverse cardiac events; PCI: percutaneous coronary intervention; SD: standard deviation
STUDY ENDPOINTS
The primary endpoint was postprocedural minimum stent area (in mm2) as assessed on the final post-procedure IVUS. As there were limited data available to estimate the expected minimum stent area after the rotational atherectomy plus cutting balloon angioplasty strategy, no formal sample size calculation was performed. The predefined total sample size of 60 patients was powered to detect effect sizes as listed in Supplementary Table 2, given an estimated minimum stent area of 5.50±2.36 mm2, an equal variance, and a two-sided alpha of 0.05.
Secondary IVUS endpoints included in-segment minimum lumen area (in mm2), minimum and mean stent expansion (in %), and any dissection and malapposition on the final IVUS. Furthermore, assessment of calcium fracture and any dissection were evaluated on IVUS post-CBA/NCBA before stent implantation. Secondary angiographic endpoints included in-segment and in-stent acute lumen gain (in mm), in-segment and in-stent final residual diameter stenosis (in %), and baseline, pre-stent and final in-segment and in-stent minimum lumen diameters (in mm). In addition, data on pre-stent and final dissections type B or greater, final perforations (Ellis type ≥II), and final side branch closures (≥1.5 mm) were collected. Device-related endpoints included any problems related to the devices, including but not limited to balloon rupture, blade detachments, difficulty withdrawing/advancing the device, or other device-related problems as identified by the investigator. Clinical endpoints at 30 days were all-cause death (further classified as cardiac and non-cardiac), myocardial infarction (as defined by the Society for Cardiovascular Angiography and Interventions [SCAI] definition10 and 4th universal definition11 including periprocedural myocardial infarction), target lesion revascularisation, target vessel revascularisation, stent thrombosis (definite/probable), major bleeding (Bleeding Academic Research Consortium [BARC] 3 or 5), and vascular complications. An independent core lab evaluated all IVUS and angiographic endpoints, and an independent clinical events committee blinded to the study treatment adjudicated all major clinical events.
QUANTITATIVE CORONARY ANGIOGRAPHY
Coronary angiography for offline quantitative coronary angiography (QCA) was performed at baseline, after RA, after CBA/NCBA, and at the end of the procedure (Central illustration).
IVUS ANALYSIS
The 60Mhz OptiCross catheter (Boston Scientific) was used for IVUS, with automated pullback at 1 mm/s. The catheter had to be advanced at least 5 mm distal to the target lesion. IVUS runs were performed at baseline (if possible), after lesion preparation was defined as RA+CBA or RA+NCBA, and at the end of the procedure (Central illustration). Any additional IVUS runs and assessments deemed clinically indicated by the operator to guide the procedure were allowed during the procedure. After the operator deemed the procedure to be finished, the final post-angioplasty IVUS run was performed for endpoint assessment. In order to reduce bias, operators were blinded to the final IVUS run pullback images, and no additional stent placement or post-dilatation was allowed after the final IVUS run.
STATISTICAL ANALYSIS
Summary statistics are presented according to treatment allocation. Categorical variables are presented as frequencies and percentages, and continuous variables are presented as means and standard deviations. Categorical variables were compared using a Chi-square test or Fisher’s exact test if Chi-square assumptions were violated. Normally distributed continuous variables were compared using the Student’s t-test, while non-normally distributed variables were assessed using a Mann-Whitney U test/Wilcoxon rank-sum test. Procedural outcomes are presented according to treatment allocation. Procedural outcomes were compared between treatment groups using a Chi-square test or Fisher’s exact test if Chi-square assumptions were violated. The probability of the occurrence of clinical outcomes up to 30 days was estimated using the Kaplan-Meier method. The hazard ratio (HR; RA+CBA compared to RA+NCBA [reference]), p-values and the corresponding 95% confidence intervals (CI) were estimated from the Cox proportional hazard model. All statistical tests were two-sided with an alpha level of 0.05 and a 95% CI.
Results
BASELINE CLINICAL CHARACTERISTICS
There were no significant differences in baseline characteristics between the RA+CBA and the RA+NCBA groups (Table 1). The mean age was 69.2±10.0 years in the RA+CBA group and 72.8±8.7 years in the RA+NCBA group (p=0.140), and the overall rate of female participants was about 22%. The burden of cardiovascular disease risk factors was high in both groups (Table 1).
Table 1. Baseline clinical characteristics.
| RA+CBA N=29 (48.3) | RA+NCBA N=31 (51.7) | p-value | |
|---|---|---|---|
| Age, years | 69.2±10.0 | 72.8±8.7 | 0.140 |
| Sex, female | 8 (27.6) | 5 (16.1) | 0.282 |
| Race | |||
| White | 12 (41.4) | 20 (64.5) | 0.073 |
| Black | 3 (10.3) | 1 (3.2) | 0.346 |
| Asian | 10 (34.5) | 5 (16.1) | 0.101 |
| Pacific Islander | 0 (0.0) | 1 (3.2) | 1.000 |
| Other | 5 (17.2) | 5 (16.1) | 1.000 |
| Hispanic/Latino | 4 (13.8) | 4 (12.9) | 1.000 |
| Hypertension | 28 (96.6) | 29 (93.5) | 1.000 |
| Hyperlipidaemia | 28 (96.6) | 29 (93.5) | 1.000 |
| Diabetes mellitus | 12 (41.4) | 13 (41.9) | 0.965 |
| Insulin | 6 (50.0) | 5 (38.5) | |
| Current smoker | 3 (11.1 ) | 6 (19.4) | 0.477 |
| Family history of CAD | 15 (75.0) | 18 (81.8) | 0.714 |
| Peripheral arterial disease | 4 (13.8) | 3 (9.7) | 0.702 |
| Cerebrovascular disease | 3 (10.3) | 5 (16.1) | 0.708 |
| Chronic renal insufficiency | 5 (17.2) | 2 (6.5) | 0.247 |
| LVEF, % | 55.6±9.8 | 57.3±7.6 | 0.453 |
| Prior CABG | 2 (6.9) | 2 (6.5) | 1.000 |
| Prior PCI | 20 (69.0) | 20 (64.5) | 0.715 |
| Prior MI | 4 (13.8) | 7 (22.6) | 0.379 |
| Prior stroke | 1 (3.4) | 2 (6.5) | 1.000 |
| Data are presented as mean±SD or n (%). CABG: coronary artery bypass grafting; CAD: coronary artery disease; CBA: cutting balloon angioplasty; LVEF: left ventricular ejection fraction; MI: myocardial infarction; NCBA: non-compliant balloon angioplasty; PCI: percutaneous coronary intervention; RA: rotational atherectomy; SD: standard deviation | |||
LESION AND PROCEDURAL CHARACTERISTICS
All patients had one lesion treated during the index procedure. A total of 10 patients (3 of the RA+CBA and 7 of the RA+NCBA group) underwent staged PCI for additional coronary lesions after the index procedure.
The procedural details of RA were similar between groups, including burr size, burr-to-artery ratio, number of burrs used and maximum burr rpm (Table 2). In the RA+CBA group, the maximum balloon length was shorter, the maximum balloon inflation pressure was lower and the number of inflations was greater, compared with the RA+NCBA group. The maximum balloon diameter and balloon-to-artery ratio of the cutting balloon and non-compliant balloon did not significantly differ. The duration time of the procedure and contrast use were also similar regardless of the treatment strategy. Post-stenting dilatation was performed in 96.6% of the RA+CBA group and in 83.9% of the RA+NCBA group (p=0.196). One patient assigned to the RA+CBA group underwent RA+NCBA.
Table 2. Lesion and procedural characteristics.
| RA+CBA N=29 (48.3) | RA+NCBA N=31 (51.7) | p-value | |
|---|---|---|---|
| Number of diseased vessels | 0.866 | ||
| 1 | 19 (65.5) | 20 (64.5) | |
| 2 | 4 (13.8) | 6 (19.4) | |
| 3 | 6 (20.7) | 5 (16.1) | |
| Target vessel | 0.895 | ||
| LAD | 18 (62.1) | 16 (51.6) | |
| Cx | 4 (13.8) | 3 (9.7) | |
| RCA | 7 (24.1) | 12 (38.7) | |
| Target vessel reference vessel diameter, mm | 3.2±0.4 | 3.3±0.5 | 0.770 |
| Procedure duration, min | 75.6±29.4 | 70.9±22.4 | 0.483 |
| Total amount of contrast used, cc | 143.4±48.0 | 146.4±38.5 | 0.792 |
| Rotational atherectomy | |||
| Burr size, mm | 1.6±0.1 | 1.6±0.2 | 0.674 |
| Burr-to-artery ratio | 0.5±0.1 | 0.5±0.1 | 0.612 |
| Number of burrs used | 1.1±0.3 | 1.0±0.0 | 0.161 |
| Number of passes over calcification | 6.2±3.4 | 6.0±3.1 | 0.775 |
| Total duration of rotational atherectomy, sec | 56.4±29.0 | 52.2±25.0 | 0.553 |
| Maximum burr, rpm | 151,931±6,058 | 151,935±3,802 | 0.997 |
| CBA/NCBA | |||
| Maximum balloon diameter, mm | 3.2±0.4 | 3.3±0.5 | 0.416 |
| Balloon-to-artery ratio | 1.0±0.1 | 1.0±0.2 | 0.633 |
| Maximum balloon length, mm | 8.7±5.1 | 20.0±6.5 | <0.001 |
| Maximum balloon inflation pressure, atm | 12.3±3.9 | 15.8±3.9 | <0.001 |
| Number of inflations | 4.2±2.5 | 2.3±0.8 | <0.001 |
| Stent | |||
| Number of stents | 0.155 | ||
| 1 | 21 (72.4) | 27 (87.1) | |
| 2 | 8 (27.6) | 4 (12.9) | |
| Maximum stent diameter, mm | 3.3±0.4 | 3.4±0.5 | 0.349 |
| Minimum stent diameter, mm | 3.2±0.4 | 3.3±0.5 | 0.203 |
| Total stent length, mm | 39.4±16.4 | 37.7±13.0 | 0.661 |
| Maximum stent inflation pressure, atm | 14.4±1.9 | 14.6±1.8 | 0.578 |
| Post-stent dilatation | |||
| Post-stent dilatation | 28 (96.6) | 26 (83.9) | 0.196 |
| Maximum balloon diameter, mm | 3.5±0.4 | 4.0±2.2 | 0.247 |
| Maximum inflation pressure, atm | 17.1±2.3 | 17.1±2.9 | 0.927 |
| Number of inflations | 3.3±2.6 | 3.0±1.7 | 0.675 |
| Values are mean±standard deviation or n (%). atm: atmosphere; CBA: cutting balloon angioplasty; cc: cubic centimeters; Cx: circumflex artery; LAD: left anterior descending artery; min: minutes; NCBA: non-compliant balloon angioplasty; RA: rotational atherectomy; rpm: rotations per minute; sec: seconds; RCA: right coronary artery | |||
PREPROCEDURAL CHARACTERISTICS AND PROCEDURAL OUTCOMES BY QCA
QCA at baseline did not show significant differences in target lesion characteristics between the groups (Table 3, Supplementary Table 3). Severe calcification was present in 72.4% of the RA+CBA group and in 74.2% of the RA+NCBA group (p=0.876). Procedural complications were rare and were related to RA rather than CBA/NCBA (Table 3, Supplementary Table 3). Post-CBA/NCBA dissections type B or greater were present in 21.4% and 44.8% of patients assigned to RA+CBA and RA+NCBA, respectively (p=0.058). One dissection was present in the RA+NCBA and none in the RA+CBA group post-procedure (after stenting±post-dilatation).
With regards to angiographic endpoints, no differences in acute lumen gain, final residual diameter stenosis or final minimum lumen diameter were found between groups in the final post-procedural assessment (Table 3).
Table 3. Preprocedural characteristics and procedural outcomes by QCA.
| RA+CBA N=29 (48.3) | RA+NCBA N=31 (51.7) | p-value | |
|---|---|---|---|
| Preprocedure | |||
| Lesion location | 0.895 | ||
| Proximal | 13 (44.8) | 14 (45.2) | |
| Mid | 15 (51.7) | 17 (54.8) | |
| Distal | 1 (3.4) | 0 (0.0) | |
| Target lesion length, mm | 31.3±11.9 | 32.8±11.2 | 0.633 |
| Minimum lumen diameter, mm | 1.1±0.4 | 1.0±0.4 | 0.348 |
| Angulation, degree | 28.8±10.9 | 29.7±8.3 | 0.725 |
| Calcification | 0.876 | ||
| Moderate | 8 (27.6) | 8 (25.8) | |
| Severe | 21 (72.4) | 23 (74.2) | |
| Calcium length, mm | 19.1±11.8 | 18.5±7.0 | 0.788 |
| Diameter stenosis, % | 62.4±11.8 | 66.8±12.5 | 0.165 |
| Post-rotational atherectomy | |||
| Dissection Type B or greater | 0 (0.0) | 4 (14.6) | N/A |
| Staining | N/A | 0 (0.0) | N/A |
| Minimum lumen diameter, mm | 1.4±0.3 | 1.4±0.4 | 0.438 |
| Post-CBA/NCBA before stent | |||
| TIMI <3 | 0 (0.0) | 0 (0.0) | N/A |
| Slow flow | 0 (0.0) | 0 (0.0) | N/A |
| No reflow | 0 (0.0) | 0 (0.0) | N/A |
| Abrupt closure | 0 (0.0) | 0 (0.0) | N/A |
| Distal embolisation | 0 (0.0) | 0 (0.0) | N/A |
| New thrombus | 0 (0.0) | 0 (0.0) | N/A |
| Perforation | 0 (0.0) | 0 (0.0) | N/A |
| Spasm | 0 (0.0) | 0 (0.0) | N/A |
| Dissection Type B or greater | 6 (21.4) | 13 (44.8) | 0.058 |
| Staining | 0 (0.0) | 2 (15.4) | N/A |
| Minimum lumen diameter, mm | 1.8±0.4 | 1.7±0.3 | 0.387 |
| Final post-procedure | |||
| Acute lumen gain, in-segment, mm | 1.0±0.4 | 1.1±0.3 | 0.370 |
| Acute lumen gain, in-stent, mm | 1.5±0.3 | 1.6±0.4 | 0.269 |
| Final residual diameter stenosis, in-segment, % | 25.8±8.6 | 28.4±6.8 | 0.203 |
| Final residual diameter stenosis, in-stent, % | 13.8±6.5 | 14.8±6.1 | 0.564 |
| Final minimum lumen diameter, in-segment, mm | 2.1±0.3 | 2.1±0.4 | 0.910 |
| Final minimum lumen diameter, in-stent, mm | 2.5±0.3 | 2.5±0.4 | 0.890 |
| Final dissection Type B or greater | 0 (0.0) | 1 (3.2) | N/A |
| Final perforation (Ellis type ≥2) | 0 (0.0) | 0 (0.0) | N/A |
| Final side branch closure | 0 (0.0) | 0 (0.0) | N/A |
| Values are n (%) or mean±standard deviation. CBA: cutting balloon angioplasty; NCBA: non-compliant balloon angioplasty; QCA: quantitative coronary angiography; RA: rotational atherectomy; TIMI: Thrombolysis in Myocardial Infarction | |||
PREPROCEDURAL CHARACTERISTICS AND PROCEDURAL OUTCOMES ON IVUS
In 45 patients, IVUS was performed at baseline, with no significant differences in preprocedural characteristics between groups (Table 4). Severe calcification was documented in 80.0% of the RA+CBA group and in 85.0% of the RA+NCBA group (p=0.519). The rate of calcium fractures was significantly higher in the RA+CBA compared with the RA+NCBA group (50.0% vs 22.6%; p=0.028). Dissections after CBA/NCBA were present in 96.4% of RA+CBA and 96.7% of RA+NCBA patients, with the majority limited to the intima and none beyond the media. In the final IVUS assessment post-procedure (after stenting±post-dilatation), dissections were present in 3.4% of the RA+CBA group versus 9.7% of the RA+NCBA group (p=0.613). Furthermore, stent malapposition was documented in 17.2% of the RA+CBA group and 29.0% of the RA+NCBA group (p=0.281).
With regards to IVUS endpoints, no significant differences between groups were found for the primary endpoint of minimum stent area (6.7±1.7 mm2 in RA+CBA vs 6.9±1.8 mm2 in RA+NCBA; p=0.685) (Central illustration, Table 4). There was also no significant difference in the minimum stent area when the medians (interquartile ranges) were compared (6.5 [5.3-7.8] mm2 in RA+CBA vs 6.9 [5.5-8.0] mm2 in RA+NCBA; p=0.636) (Central illustration). Furthermore, no significant difference was noted for the in-segment minimum lumen area between groups. The final minimum and mean stent expansion tended to be larger in RA+CBA versus RA+NCBA patients (86.1±17.5% vs 78.6±14.7% and 112.5±19.4% vs 104.2±13.5%, respectively); however, the differences did not reach statistical significance (p=0.076 and p=0.058, respectively).
Table 4. Preprocedure characteristics and procedural outcomes on IVUS.
| RA+CBA N=29 (48.3) | RA+NCBA N=31 (51.7) | p-value | |
|---|---|---|---|
| Preprocedure | |||
| Target lesion length, mm | 32.2±9.6 | 36.0±10.8 | 0.286 |
| Minimum lumen area, mm2 | 2.4±0.8 | 2.5±0.6 | 0.612 |
| Eccentricity | 15 (100) | 20 (100) | N/A |
| Thrombus | 0 (0) | 0 (0) | N/A |
| Calcification | 0.519 | ||
| Moderate | 3 (20.0) | 3 (15.0) | |
| Severe | 12 (80.0) | 17 (85.0) | |
| Calcium length, mm | 26.0±7.6 | 29.4±9.6 | 0.256 |
| Calcium arc max, degrees | 319.3±61.2 | 303.5±63.7 | 0.467 |
| Post-CBA/NCBA before stent | |||
| Calcium fracture | 14 (50.0) | 7 (22.6) | 0.028 |
| Dissection | 27 (96.4) | 30 (96.7) | 1.000 |
| Length, mm | 7.8±5.3 | 8.5±6.3 | 0.514 |
| Depth | 0.172 | ||
| Intimal | 25 (86.2) | 24 (77.4) | |
| Medial | 2 (6.9) | 6 (19.3) | |
| Adventitial | 0 (0) | 0 (0) | |
| Final post-procedure | |||
| Minimum stent area, mm2 | 6.7±1.7 | 6.9±1.8 | 0.685 |
| In-segment minimum lumen area, mm2 | 5.9±1.4 | 6.4±1.9 | 0.291 |
| Final minimum stent expansion, % | 86.1±17.5 | 78.6±14.7 | 0.076 |
| Final mean stent expansion, % | 112.5±19.4 | 104.2±13.5 | 0.058 |
| Dissection | 1 (3.4) | 3 (9.7) | 0.613 |
| Length, mm | N/A | 1.6±0.2 | N/A |
| Depth | 1.000 | ||
| Intimal | 1 (3.4) | 2 (6.4) | |
| Medial | 0 (0) | 1 (3.2) | |
| Adventitial | 0 (0) | 0 (0) | |
| Stent malapposition | 5 (17.2) | 9 (29.0) | 0.281 |
| Values are mean±standard deviation or n (%). CBA: cutting balloon angioplasty; IVUS: intravascular ultrasound; NCBA: non-compliant balloon angioplasty; RA: rotational atherectomy. | |||
DEVICE-RELATED COMPLICATIONS
No device-related complications − including balloon rupture, blade detachment, difficulty withdrawing/advancing the device, or other device-related problems as identified by the investigator − occurred in either of the groups (Supplementary Table 4).
CLINICAL OUTCOMES AT 30 DAYS
In the intention-to-treat population, two myocardial infarctions occurred in the RA+CBA group and one in the RA+NCBA group (6.9% vs 3.2%; HR 2.14, 95% CI: 0.19-23.6; p=0.535) (Table 5). All of the myocardial infarctions were type 4a myocardial infarctions, based on the 4th universal definition11, associated with the index procedure. One of the patients with myocardial infarction and the only patient with a target vessel revascularisation was the crossover patient that received RA+NCBA instead of the assigned RA+CBA, and therefore, the clinical endpoint analyses in the per-protocol and as-treated populations found slightly different results (Supplementary Table 5, Supplementary Table 6); however, there were no significant differences between groups.
Table 5. 30-day clinical outcomes (intention-to-treat population).
| Outcomes | RA+CBA N=29 (48.3%) | RA+NCBA N=31 (51.7%) | Hazard ratio (95% CI) | p-value |
|---|---|---|---|---|
| no. of events (%) | ||||
| All-cause death | 0 (0.0%) | 0 (0.0%) | N/A | N/A |
| MI | 2 (6.9%) | 1 (3.2%) | 2.14 (0.19-23.6) | 0.535 |
| TVR | 1 (3.4%) | 0 (0.0%) | N/A | N/A |
| TLR | 0 (0.0%) | 0 (0.0%) | N/A | N/A |
| Stent thrombosis | 0 (0.0%) | 0 (0.0%) | N/A | N/A |
| Major bleeding (BARC 3 or 5) | 0 (0.0%) | 0 (0.0%) | N/A | N/A |
| Vascular complications | 0 (0.0%) | 0 (0.0%) | N/A | N/A |
| The percentages mentioned above represent Kaplan-Meier rates at 30 days after index procedure. BARC: Bleeding Academic Research Consortium; CBA: cutting balloon angioplasty; CI: confidence interval; MI: myocardial infarction; N/A: not applicable; NCBA: non-compliant balloon angioplasty; no.: number; RA: rotational atherectomy; TVR: target vessel revascularisation; TLR: target lesion revascularisation | ||||
Discussion
ROTA-CUT is a randomised controlled trial to evaluate the safety and efficacy of lesion preparation with a combination of RA+CBA versus RA+NCBA in patients undergoing PCI with drug-eluting stent implantation of moderately or severely calcified lesions. The primary endpoint, minimum stent area assessed on IVUS, was not significantly different between groups. Similarly, secondary IVUS outcomes, including in-segment minimum lumen area and stent expansion, did not significantly differ between groups. With respect to secondary angiographic endpoints, acute lumen gain, final residual diameter stenosis, and final minimum lumen diameter were also similar between groups. Overall, a strategy of RA followed by CBA was safe with rare procedural complications related to RA rather than CBA. No device-related complications occurred, and clinical endpoints were few at 30 days, with no significant differences between groups.
Lesion preparation for PCI of moderately or severely calcified coronary lesions remains a challenge for interventionalists despite the continuous development of new devices and techniques and multiple investigations on the efficacy and safety of different strategies to facilitate stent deployment and expansion121314. With regard to atheroablative strategies, RA can effectively modify calcified plaque and smooth the vessel lumen to enable subsequent balloon dilatation and stent implantation715. The ROTAXUS trial investigated lesion preparation with RA compared to standard balloon dilatation in patients with moderately or severely calcified coronary lesions8. Despite higher strategic success and initially higher acute lumen gain with RA, late lumen loss was higher with RA, and MACE rates were similar between groups at 9 months. In the PREPARE-CALC Trial, RA was compared with atherotomy using scoring or cutting balloons in patients with severe calcification16. RA was associated with a significantly higher strategy success rate than atherotomy, with comparable late lumen loss at 9 months. The ROTA-CUT trial is a randomised comparison (under FDA IDE) of combining an atherectomy with an atherotomy strategy by applying RA followed by CBA versus RA followed by NCBA. Our results support the feasibility and safety of such a strategy with only one crossover to the RA+NCBA group and low complication rates that are related to RA rather than CBA. In the RA+CBA group, slow flow (7.1%), no reflow (3.6%) and distal embolisation (7.1%) were present after RA, but none of these complications occurred after CBA. A high prevalence of dissections was present on IVUS after RA+CBA as well as after RA+NCBA, but most dissections were limited to the intima, and no dissection was noted beyond the media in either of the groups. On final IVUS, only one dissection was present in the RA+CBA group. The significantly higher rate of calcium fractures in the RA+CBA group compared with the RA+NCBA group did not translate into greater minimum stent area or acute lumen gain on final IVUS and QCA, respectively. Following on from an earlier pilot study17, the recently published PREPARE-CALC-COMBO study investigated the combination of RA and CBA for lesion preparation in patients with severely calcified lesions18. The two primary endpoints of the PREPARE-CALC-COMBO study were in-stent acute lumen gain by QCA and stent expansion on optical coherence tomography (OCT). In contrast to our results, the PREPARE-CALC-COMBO investigators found a higher acute lumen gain and a larger minimum stent area with the RA+CBA strategy compared with RA or scoring/cutting balloon angioplasty alone. Stent expansion on OCT was comparable between the 3 groups. Interestingly, the degree of stent expansion with the RA+CBA strategy was higher in ROTA-CUT compared to PREPARE-CALC-COMBO (86.1±17.5% vs 75.1±13.8%) and more favourable compared to the RA+NCBA group within the study, although the latter difference did not reach statistical significance. Noticeable differences between ROTA-CUT and PREPARE-CALC-COMBO should be acknowledged when comparing the results of both studies. First, ROTA-CUT was a randomised controlled trial, and PREPARE-CALC-COMBO was a prospective single-arm study comparing results with a historical cohort (the randomised PREPAREâCALC trial), thereby introducing potential operator bias. Second, the primary endpoints differed between studies. Several previous studies have suggested minimum stent area on IVUS to be the most powerful predictor for future adverse events192021. However, a recent study investigating alternative models to assess stent expansion on IVUS suggests that the ratio of stent area to vessel area at the minimum stent area is superior to absolute minimum stent area in predicting target lesion revascularisation or stent thrombosis, especially in a cohort with overall excellent final minimum stent area22. Analyses according to alternative models of stent expansion were performed but were not included in the results section of this manuscript; see Supplementary Table 7. Cutoff values to predict stent failure have been suggested as 5 to 5.5 mm22324. Third, in ROTA-CUT, CBA was performed according to the instructions for use, with an average maximum cutting balloon inflation pressure of 12.3±3.9 atm compared with 16.9±2.7 atm in PREPARE-CALC-COMBO. Lastly, ROTA-CUT used IVUS imaging for endpoint assessment, while PREPARE-CALC-COMBO used OCT. Final IVUS imaging was available in all patients in the ROTA-CUT trial, while OCT assessment in PREPARE-CALC-COMBO was missing in about 30%. Importantly, the final IVUS in ROTA-CUT was blinded to the operator so that the operator had to rely on the angiographic result for completion of the procedure. Previous data have suggested using IVUS-optimised stent implantation in complex coronary lesions, rather than angiographic guidance only, but this results in a larger minimum lumen diameter25. More recent data have added to the evidence supporting the important role of intravascular imaging guidance throughout complex coronary interventions, including data suggesting a larger minimum stent area with OCT versus angiography guidance26. Nevertheless, whether access to the final IVUS run for operators would have impacted the study results remains uncertain.
The findings of the present study have important implications. The use of a combination of RA and CBA appears safe in clinical practice and is associated with high procedural success. These findings may be useful to the operator if RA alone does not result in the desired plaque modification. In addition, the evidence provided by ROTA-CUT has set the stage for larger trials to evaluate a strategy of RA followed by CBA for improvement of stent expansion in patients undergoing PCI for moderately or severely calcified coronary lesions.
Limitations
Certain limitations of the present trial have to be acknowledged when interpreting the results. First, ROTA-CUT was conducted in 2 high-volume tertiary PCI centres with a high prevalence of calcific coronary artery disease patients and highly experienced operators. The high procedural success rate may not be replicated in less experienced centres. Second, the sample size was small, and definitive conclusions based on the results may be limited considering the possibility of a type 2 error. However, the findings of ROTA-CUT, based on serial IVUS and angiographic assessments, will inform the design and conduct of larger trials on strategies to improve stent expansion in patients undergoing PCI for moderately or severely calcified coronary lesions. Third, in addition to lesions with severe calcification, lesions with moderate calcification were included in the ROTA-CUT trial. The advantage of using a cutting balloon in addition to RA might, however, only become apparent in a population with severely calcified lesions. This may be a consideration for the design of future trials. Fourth, post-stent dilatation was optional and was more often performed in the RA+CBA group (96.6%) compared with the RA+NCBA group (83.9%). Although this difference was not statistically significant (p=0.196), its impact on the study results remains uncertain. Lastly, although follow-up for clinical outcomes at 270 days is ongoing, no further angiographic follow-up will occur, and evaluating the impact of treatment strategy on late lumen loss will not be possible.
Conclusions
A strategy of combining RA with cutting balloon angioplasty resulted in a similar minimum stent area compared with RA followed by non-compliant balloon angioplasty in patients undergoing PCI of moderately or severely calcified lesions. RA followed by cutting balloon angioplasty for the preparation of moderately or severely calcified lesions was safe with rare procedural complications and few clinical adverse events at 30 days.
Impact on daily practice
The significantly higher rate of calcium fractures in patients undergoing rotational atherectomy (RA) and cutting balloon angioplasty (CBA) compared with patients undergoing RA followed by non-compliant balloon angioplasty (NCBA) did not translate into a greater minimum stent area or acute lumen gain. However, RA followed by cutting balloon angioplasty for the preparation of moderately or severely calcified lesions was safe with rare procedural complications and few clinical adverse events at 30 days. Also, the final minimum and mean stent expansion tended to be larger in the RA+CBA versus RA+NCBA group, although the differences did not reach statistical significance. These findings may be useful to the operator if RA alone does not result in the desired plaque modification.
Funding
The study was supported by an investigator-initiated grant from Boston Scientific.
Conflict of interest statement
R. Mehran reports institutional research payments from Abbott, Abiomed, Affluent Medical, Alleviant Medical, Amgen, AM-Pharma, Applied Therapeutics, Arena, AstraZeneca, AtriCure, Biosensors, Biotronik, Boston Scientific, Bristol-Myers Squibb, CardiaWave, CeloNova, Chiesi, Concept Medical, CSL Behring, CytoSorbents, Daiichi Sankyo, Duke, Element Science, Faraday, Humacyte, Idorsia, I-Laser, Janssen, Magenta Medical, MedAlliance, Medscape, MediaSphere Medical, Medtelligence, Medtronic, MJH Healthcare, Novartis, OrbusNeich, Penumbra, PhaseBio, Philips, Pi-Cardia, PLx Pharma, Protembis, RenalPro, RM Global, Shockwave Medical, Transverse Medical, Vivasure Medical, and ZOLL Medical; personal fees from Affluent Medical, Cardiovascular Research Foundation (CRF), Daiichi Sankyo Brasil, E.R. Squibb & Sons, Esperion Science, Innovative BioPharma, Europa Group, Boston Scientific, Gaffney Events, Educational Trust, Ionis Pharmaceuticals, jCalc, Novartis, Novo Nordisk, Vectura, Voxmedia LLC, IQVIA, McVeigh Global Meetings and Events, Overcome, Primer Healthcare of New Jersey, Radcliffe, SL Solutions, Tarsus Cardiology, and WebMD; equity <1% in Applied Therapeutics, Elixir Medical, Stel Life, and ControlRad (spouse); no fees from AMA (Scientific Advisory Board), or SCAI (Women in Innovations Committee Member); faculty member of CRF; and honoraria from JAMA Cardiology (Associate Editor) and ACC (BOT Member, SC Member CTR Program). Z.A. Ali reports institutional grant support from Abbott, Abiomed, ACIST Medical, Amgen, Boston Scientific, CathWorks, Canon, Conavi Medical, HeartFlow, Inari Medical, Medtronic, National Institute of Health, Nipro, OpSens Medical, Medis, Philips, Shockwave Medical, Siemens, SpectraWAVE, and Teleflex; consulting fees from Abiomed, AstraZeneca, Boston Scientific, CathWorks, OpSens, Philips, and Shockwave Medical; and equity in Elucid, Lifelink, SpectraWAVE, Shockwave Medical, and VitalConnect. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

