
Abstract
Aims: We aimed to evaluate whether optical coherence tomography (OCT)-guided rotational atherectomy (RA) improves stent expansion and clinical outcomes compared to intravascular ultrasound (IVUS)-guided RA.
Methods and results: From our database, we identified 247 de novo calcified coronary lesions that underwent RA between September 2013 and December 2017. Of these, lesions with no intravascular imaging data (n=11), poor image quality (n=7), balloon angioplasty alone (n=16), and complications (two burr entrapments, two perforations) were excluded. Finally, 88 and 121 lesions that underwent OCT-guided and IVUS-guided RA, respectively, were included in the study. The primary endpoint of the present study was percent stent expansion. Burr upsizing was more frequently performed (55% vs 32%, p=0.001) and the final burr size was significantly larger (1.75 [1.50-1.75] vs 1.50 [1.50-1.75] mm, p<0.001) in the OCT-guided RA group. Percent stent expansion was significantly larger in the OCT-guided RA group (83±15% vs 72±16%, p=0.0004). Although TLR at one year was lower in the OCT-guided RA group, there was no statistical difference (6.8% vs 11.6%, p=0.25).
Conclusions: OCT-guided RA for calcified coronary lesions resulted in larger percent stent expansion compared to IVUS-guided RA. OCT-guided RA may be ideal for treating calcified coronary lesions.
Introduction
Severely calcified lesions remain challenging to treat with percutaneous coronary intervention (PCI). Severe calcification is commonly associated with procedural complications, balloon delivery failure, stent underexpansion, and a high incidence of target lesion revascularisation (TLR)1,2. Lesion modification using rotational atherectomy (RA) facilitates optimal stent implantation and reduces major adverse cardiac events (MACE) at follow-up3. Intravascular ultrasound (IVUS)-guided PCI is known to be associated with better clinical outcomes compared to angiography-guided PCI4. However, IVUS cannot provide detailed quantitative assessment of calcifications because ultrasound cannot penetrate calcium. Optical coherence tomography (OCT) has a higher resolution than IVUS and can visualise calcium distribution clearly without an acoustic shadow5. To achieve maximum drug-eluting stent (DES) performance for severely calcified lesions, selecting the optimal RA endpoint is important. We aimed to evaluate how OCT-guided RA affects PCI, including the selection of burr size for RA, stent expansion, and whether restenosis is reduced, compared with IVUS-guided RA for calcified coronary lesions.
Methods
STUDY PROTOCOL AND SUBJECTS
This study was a single-centre retrospective observational study. Between September 2013 and December 2017, we performed RA for 247 de novo calcified coronary lesions. The indication for RA was lesions with >180° arc of calcium assessed by intracoronary imaging or lesions with angiographic calcification where an imaging catheter could not pass through. Although the intravascular imaging devices used were at each operator’s discretion, OCT-guided RA was preferred except in renal dysfunction (serum creatinine >2.0 mg/dl), ostial lesions with challenging blood removal, and tortuous calcified lesions in which OCT catheter delivery appeared difficult. The study protocol conformed to the Declaration of Helsinki and was approved by the institutional review board of our hospital. All patients provided written informed consent regarding the procedures and collection and analysis of anonymised data for research purposes.
PROCEDURE AND FOLLOW-UP
All patients received a bolus injection of heparin (5,000 IU) and the activated coagulation time was maintained at >300 s with an additional bolus of heparin. Dual antiplatelet therapy with 100 mg/day aspirin and either 75 mg/day clopidogrel or 3.75 mg/day prasugrel was continued for at least one year post procedure. Initially, we evaluated the severity of calcification using intravascular imaging. A frequency-domain OCT system (ILUMIEN™ OPTIS™; Abbott Vascular, Santa Clara, CA, USA) or optical frequency domain imaging (OFDI) system (Lunawave®; Terumo, Tokyo, Japan) was used for OCT-guided RA, and an IVUS system (iLab™; Boston Scientific, Marlborough, MA, USA, or VISIWAVE™; Terumo) was used for IVUS-guided RA. We used the ROTABLATOR™ Rotational Atherectomy System (Boston Scientific) for lesion atherectomy in all cases. The conventional 0.014-inch guidewire was replaced with the 0.009-inch ROTAWire™ Floppy guidewire (Boston Scientific) or ROTAWire™ Extra Support guidewire (Boston Scientific) at the operator’s discretion. We used up to two burr sizes in the procedure. The initial burr size was chosen based on the preprocedural intravascular imaging findings and, when the imaging catheter could not pass the lesion, we selected either a 1.25 or 1.5 mm burr based on the angiographic vessel size. Burr speed was selected at 220,000 rpm, with a run duration of 10–15 s, or less in cases where there was a drop of >5,000 rpm. After initial atherectomy, we performed intravascular imaging again and decided whether burr upsizing was needed. Representative burr selection in OCT-guided RA is shown in Figure 1. In OCT-guided RA, we measured the thickness of the calcification at the smallest cross-sectional area (CSA) with the greatest extent of calcification and decided whether burr upsizing was needed. Operators tried to make a thin part of the calcification, so that it could crack with subsequent balloon dilatation; however, the target thickness of calcification was dependent on each operator’s discretion. Stent diameter was determined by measuring the external elastic lamina diameter at the proximal and distal reference sites. We determined stent length by measuring the distance from the distal to the proximal reference sites. Figure 2 shows a representative case of IVUS-guided RA. In-IVUS guided RA, we were unable to measure calcium thickness; therefore, we estimated the need for burr upsizing depending on the minimum lumen CSA, whether crack formation occurred after RA, and whether the vessel behind the calcification was viewable. Stent diameter was determined by measuring the external elastic lamina at the proximal and distal reference sites. Stent length was determined as in OCT-guided RA. We performed a final intracoronary imaging after stent implantation with subsequent post-dilatation. Patients were followed up at our outpatient clinic, and clinical data were obtained from hospital records and by telephone interview when patients did not attend the outpatient clinic.
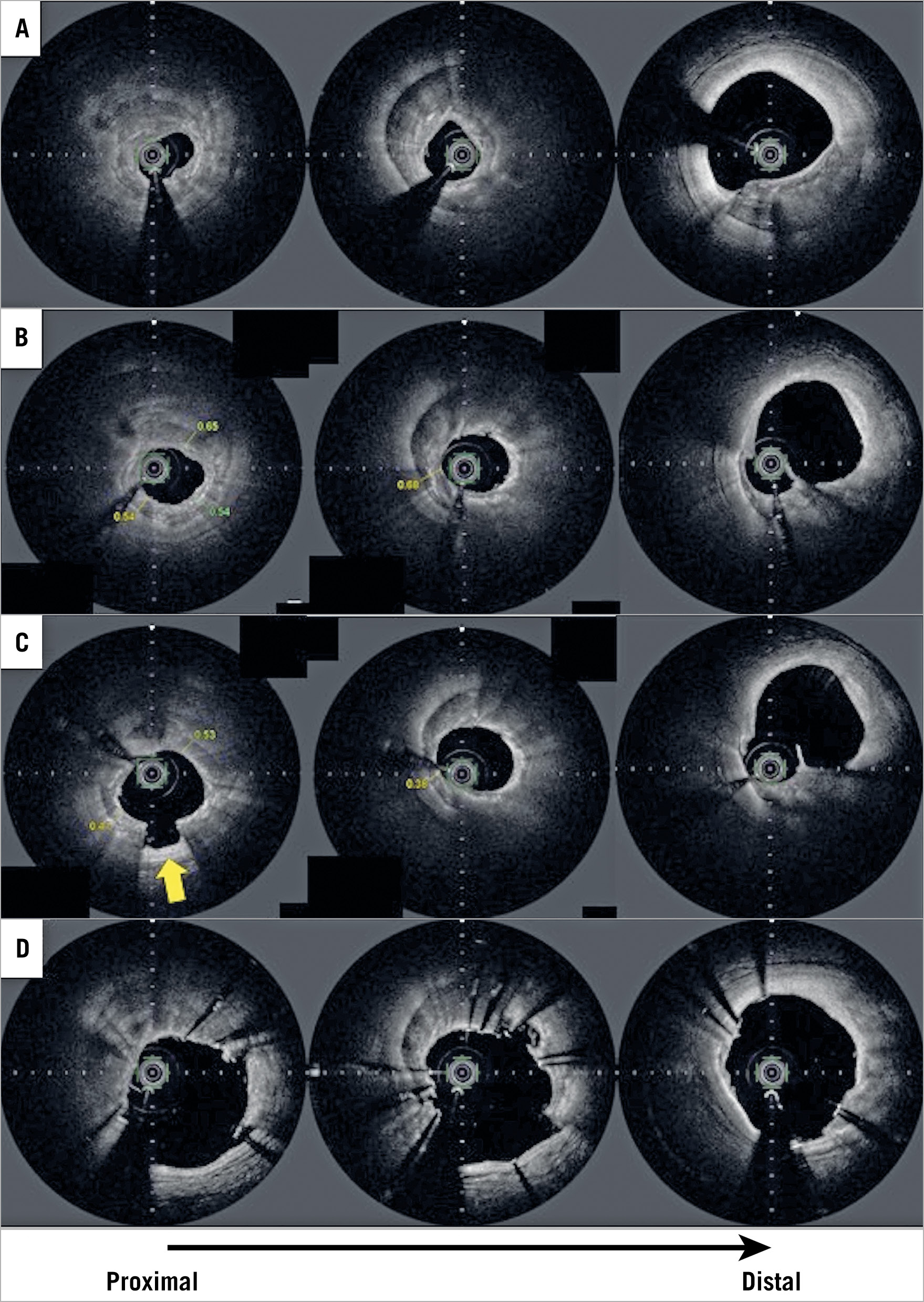
Figure 1. Representative case of rotational atherectomy (RA) with optical frequency domain imaging (OFDI). A) OFDI preprocedural image showing severe, thick calcium with a 360° calcium arc. B) OFDI image after RA with a 1.75 mm burr. The thinnest part of the calcium was 540 μm; however, a large volume of the calcium remained, and a considerable part was thick. The operator decided to upsize the burr. C) OFDI image after additional RA with a 2.0 mm burr. The thinnest part of the calcium became <500 μm and some parts of the calcium cracked (arrow). D) Final OFDI image after stent implantation with subsequent post-dilatation. There were some malapposed struts; however, the stent expansion rate was acceptable (87.6%).
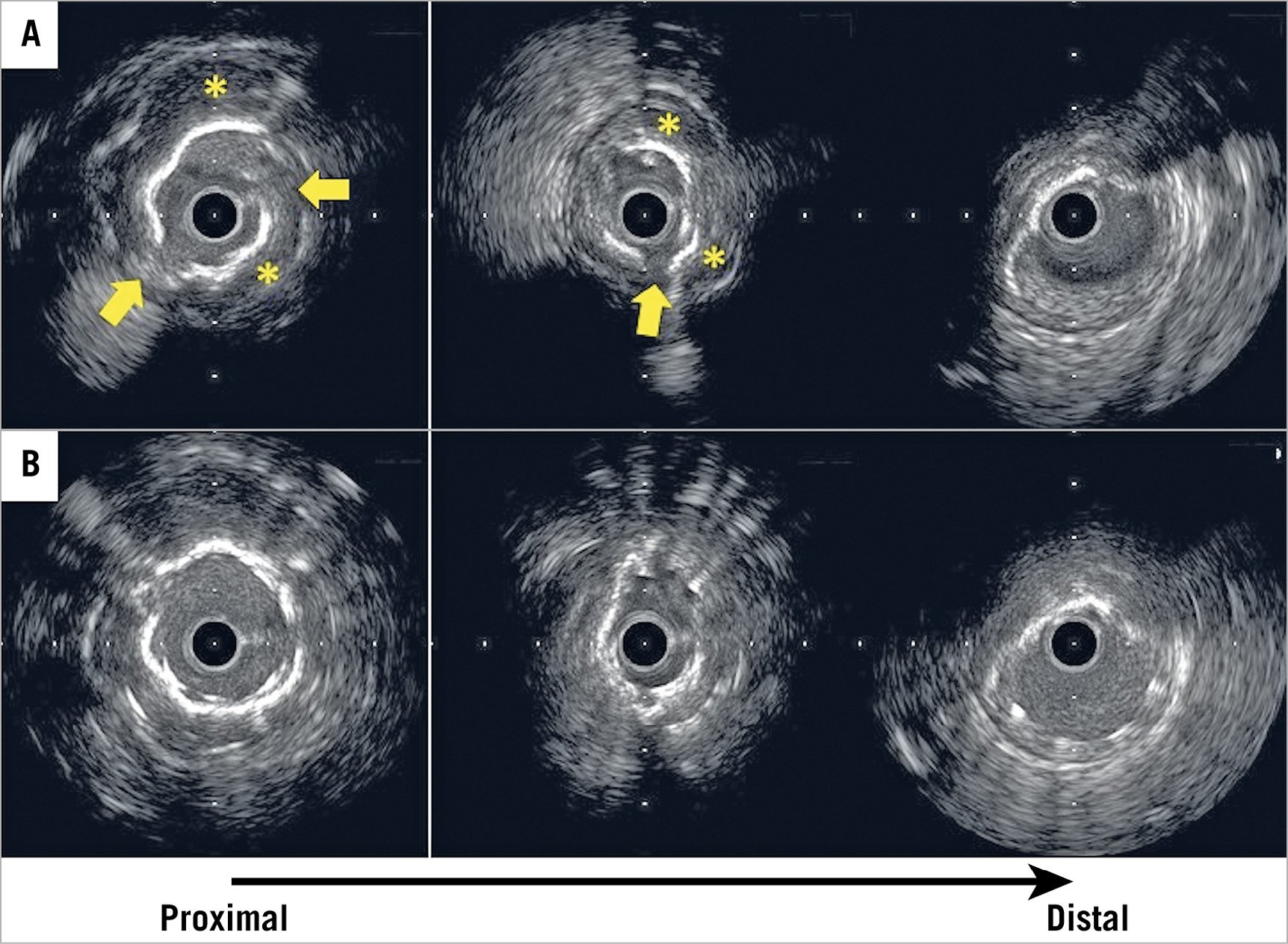
Figure 2. Representative case of rotational atherectomy (RA) with intravascular ultrasound (IVUS). A) IVUS could not cross the lesion, so RA with a 1.5 mm burr was performed. Subsequent IVUS image showed cracks at some parts of the calcium (arrows). Moreover, behind, a high echo band with acoustic shadow could be seen (asterisk); therefore, the operator considered that the calcium was not notably thick. The operator did not perform burr upsizing in this case. B) IVUS image after stent implantation with subsequent post-dilatation. Stent underexpansion was not improved even after post-dilation with high pressure. The stent expansion rate was 62.6%.
QUANTITATIVE CORONARY ANGIOGRAPHY
Quantitative coronary angiography (QCA) analysis was performed preprocedurally and post-procedurally using computer-based software (Heart II ver 2.0.2.3; Gadelius, Tokyo, Japan) by an experienced independent operator who was unaware of the patient characteristics and study objectives. Optimal views of the lesions were obtained at baseline, and the same projection angle was used after stenting.
INTRACORONARY IMAGE ACQUISITION AND ANALYSIS
In OCT-guided RA, the OCT catheter (Dragonfly™ OPTIS™; Abbott Vascular) or OFDI catheter (FastView®; Terumo) was advanced distal to the lesion, and contrast medium was injected during automatic pullback of the catheter at a rate of 20 mm/s. OCT images were analysed using an offline review workstation (LightLab Imaging, Inc./Abbott Vascular) or offline OFDI analysis software (Lunawave Offline Viewer; Terumo). In IVUS-guided RA, the IVUS catheter (OptiCross™; Boston Scientific, or ViewIT®; Terumo) was located at the distal site of the lesions and automatically pulled back at a rate of 1.0 mm/s. IVUS images were analysed using computerised planimetry software (echoPlaque; INDEC Medical Systems, Los Altos, CA, USA). In OCT or OFDI images, calcification was defined as signal-poor or heterogeneous regions with sharply delineated borders6. In IVUS images, calcification was defined as bright echoes with acoustic shadowing. All images were independently assessed by two investigators who were blinded to patient and clinical data. A third reviewer resolved any discordance. All intravascular images after stent implantation were analysed at 1 mm intervals within the stented segment. Minimum stent area was measured in each stent. The proximal and distal references were set at sites with the largest lumen area within 10 mm of the stenosis6. The mean reference lumen CSA was defined as the average lumen CSA at the proximal and distal reference sites. Percent stent expansion was defined as minimum stent area divided by the mean reference lumen CSA. Qualitative analysis regarding stent malapposition, stent edge dissection, and tissue protrusion was performed in every frame7,8. Stent malapposition was considered when the distance between the centre reflection of the stent and the vessel wall was greater than stent thickness plus the OCT resolution limit (20 μm). Stent edge dissection was defined as arterial disruption adjacent to the stent where a flap of tissue could be clearly differentiated from the underlying plaque. Tissue protrusion was defined as the prolapse of tissue through the stent struts into the lumen.
ENDPOINTS
The primary endpoint was percent stent expansion at the time of final intravascular imaging evaluation. As OCT measurements are smaller than IVUS measurements8, percent stent expansion was chosen as a primary endpoint to compare the stent expansion between the two groups in the present study with reference to a previous study9. Major secondary endpoints included the rate of burr upsizing in RA, final burr size in RA, and TLR at one year.
STATISTICAL ANALYSIS
Categorical data are presented as counts and percentages and were compared using Fisher’s exact test. Continuous variables with normal distributions are presented as means±standard deviations and were compared using an unpaired t-test. Continuous variables without normal distributions are expressed as the median and interquartile range and were compared using the Mann-Whitney U test. The reproducibility of percent stent expansion was assessed in 20 randomly selected patients. Intra- and inter-observer agreement was evaluated after the same observer and another experienced reader repeated the analysis using intraclass correlation coefficients (ICCs). Multivariable linear regression analysis was used to test whether differences in percent stent expansion remained significant after adjusting for diabetes, haemodialysis, acute coronary syndrome, and bifurcation. All probability values were two-sided and p<0.05 was considered statistically significant. All statistical analyses were conducted using SPSS, Version 19 (IBM Corp., Armonk, NY, USA).
Results
PATIENT ENROLMENT
Of 247 de novo lesions that underwent RA, we excluded lesions with no intravascular imaging data (n=11), poor image quality (n=7), balloon angioplasty alone (n=16), and complications (two burr entrapments, two perforations). Finally, 88 and 121 de novo calcified lesions that underwent OCT/OFDI-guided RA and IVUS-guided RA, respectively, were included in the study.
BASELINE CHARACTERISTICS AND PROCEDURE RESULTS
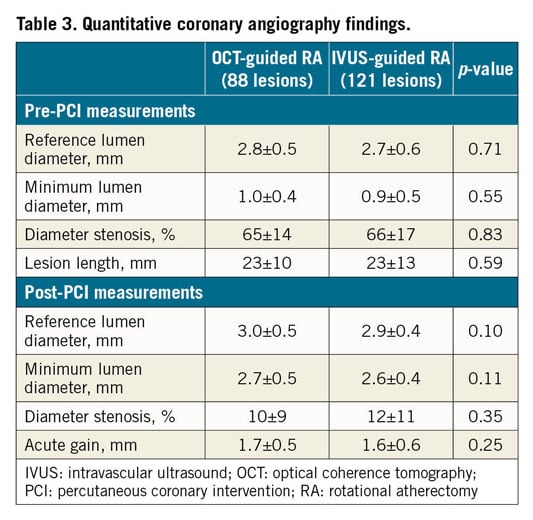
The baseline characteristics were similar between the groups except for the significantly more frequent prescription of prasugrel in the OCT-guided RA group (19% vs 5%, p=0.001) (Table 1). Regarding lesion characteristics, there was no significant difference between the groups. Procedure results are summarised in Table 2. Burr upsizing was more frequent (55% vs 32%, p=0.001) and the final burr size was significantly larger in the OCT-guided RA group (1.75 [1.50-1.75] vs 1.50 [1.50-1.75] mm, p<0.001). There was no significant difference regarding pre- and post-balloon dilatation between the groups. OCT-guided RA required a larger volume of contrast media; however, it did not induce exacerbation of renal function in non-haemodialysis patients (Table 1).
QUANTITATIVE CORONARY ANGIOGRAPHY FINDINGS
There was no difference in pre- and post-procedure QCA measurements between the groups (Table 3).
INTRAVASCULAR IMAGING FINDINGS
The OCT-guided RA group had significantly smaller quantitative measurements in the proximal reference and lesion sites before stenting compared to the IVUS-guided RA group (Table 4). However, minimum stent CSA was similar between the groups. As a result, percent stent expansion was significantly larger in the OCT-guided RA group compared to the IVUS-guided RA group (83±15% vs 72±16%, p=0.0004) (Figure 3). This remained significant after adjusting for diabetes, haemodialysis, acute coronary syndrome, and bifurcation (adjusted p-value=0.0009). The intra- and inter-observer agreement for percent stent expansion was 0.90 and 0.87, respectively.
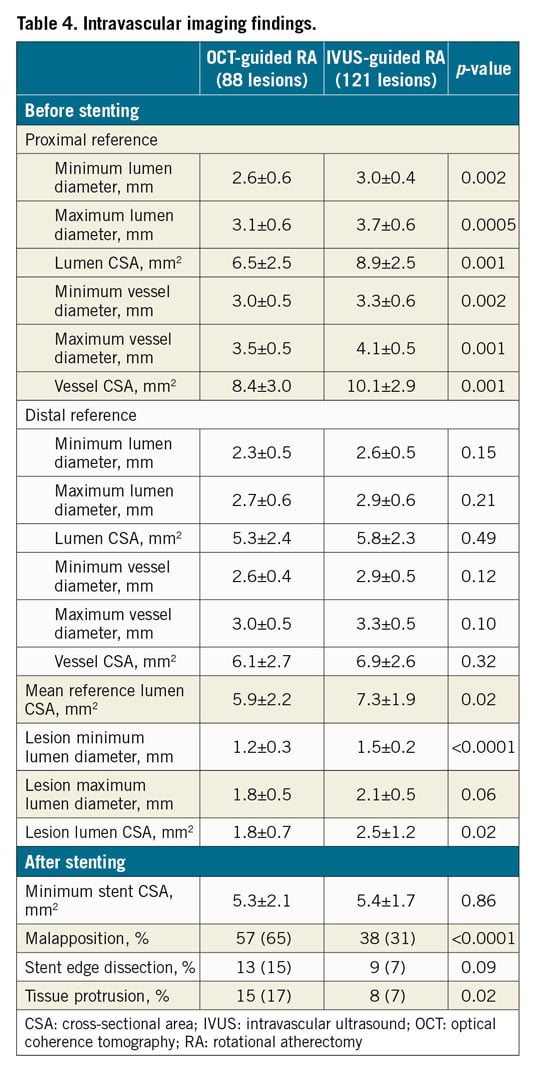
Figure 3. Comparison of stent expansion rates between the groups.
CLINICAL OUTCOMES
One-year clinical follow-up was achieved similarly in both groups (97% in the OCT-guided RA group vs 96% in the IVUS-guided RA group, p=0.79). TLR at one year was lower, but non-significantly, in the OCT-guided RA group (6.8% vs 11.6%, p=0.25) (Figure 4).
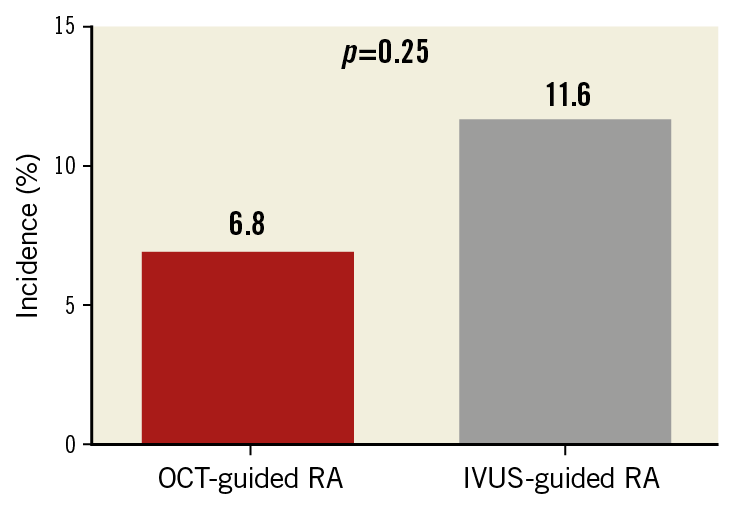
Figure 4. Target lesion revascularisation at one year between the groups.
Discussion
Previous studies have demonstrated that aggressive burr size selection (burr to artery ratio of >0.7) was not associated with improved clinical outcomes and had a high incidence of complications10,11. These studies suggested that lesion modification with a small burr size (burr to artery ratio ≤0.7) was a reasonable strategy for calcified lesions. However, while angiography provides good location of calcium in the lesion, it is not the preferred modality for burr size selection. Intravascular imaging helps the operator to determine which part of the lesion should be thoroughly ablated due to heavy calcium and the part of the lesion that should not be ablated with a large burr based on the wire bias or lipid-rich plaque location. We consider that intravascular imaging-guided RA is useful for obtaining maximum calcium ablation and preventing complications.
Several studies have shown that OCT-guided PCI is comparable to IVUS-guided PCI9,12. To our knowledge, this is the first study comparing OCT-guided and IVUS-guided RA for calcified coronary lesions with respect to burr selection difference, stent expansion, and clinical outcomes. IVUS is useful for localising the calcium but is limited in evaluating calcium thickness because of high-intensity reflection with acoustic shadow. OCT can penetrate calcium, allowing the determination of remnant calcium thickness after initial RA. The thin part of the calcium is associated with crack formation after balloon angioplasty and is related to stent expansion13,14,15. A previous study showed that the minimum thickness of the calcium was significantly correlated with stent expansion, while the maximum thickness of the calcium was not14. Furthermore, Maejima et al suggested that the optimal cut-off point of calcium thickness for obtaining a crack after ballooning is 670 μm13. In another study, the median calcium thickness for obtaining a crack was 450 μm15. Routine burr upsizing based on angiographic findings is arbitrary and can even be harmful; however, OCT findings after initial atherectomy will provide information as to whether lesion modification after initial RA is inadequate and if burr upsizing is appropriate to obtain more optimal lesion modification and facilitate good stent expansion. In IVUS-guided RA, we usually consider the necessity of burr upsizing according to the minimum lumen CSA, presence of crack, and the visibility of the vessel behind the calcification. However, these criteria cannot reliably guarantee stent expansion, as shown in our representative case. Thus, OCT-guided RA seems a better strategy than IVUS-guided RA, and our results suggested larger stent expansion with OCT-guided RA.
Although high rates of restenosis and TLR within one year after RA with DES implantation have been reported (11.3-18.6%)3,16,17, our TLR rate at one year with OCT-guided RA (6.8%) was better than that of previous studies. Jinnouchi et al17 reported that second-generation DES after RA improved the TLR rate at one year compared to first-generation DES (13.4% vs 25.2%, p=0.047). Although the incidence was not clearly described, they also used IVUS during the procedure; however, OCT was not used. We expect that OCT-guided RA with subsequent implantation of second- and third-generation DES may improve clinical outcomes for challenging calcified lesions.
Study limitations
There were several limitations to the present study. First, it was a retrospective observational study with a small sample size. Second, the number of excluded lesions was relatively large; therefore, selection bias may have existed. Third, lesions that underwent IVUS-guided RA might have been more complex compared to those that underwent OCT-guided RA. The IVUS catheter has superior crossability than the OCT catheter; therefore, operators may have preferred IVUS for severely tortuous and large calcified lesions. In addition, OCT involves the injection of contrast media, so IVUS may be preferred for patients with renal insufficiency who usually have more complex lesions. These potential biases may have been associated with more challenging stent expansion in IVUS-guided RA. Fourth, the PCI protocol, including burr size selection, balloon dilatation strategy, and stent selection, was not strictly determined because of the retrospective design. Inter-operator differences in technical and therapeutic strategies might have affected outcomes. Fifth, the primary endpoint of the present study was stent expansion, which is not a widely accepted endpoint. In addition, stent expansion was evaluated by different modalities. This study was retrospective in nature; therefore, we could not optimise this issue. In addition, the small sample size may be associated with a smaller area at the proximal reference site in the OCT-guided group. This might be associated with better stent expansion in the OCT-guided group. Sixth, in the OCT-guided group, the target thickness of the calcification was unclear. During the study period, several studies reported a cut-off point of calcium thickness for obtaining a crack after ballooning13,15. This limitation should be assessed in other prospective studies. Finally, the difference in TLR at one year between the groups may have been underpowered because of the small sample size. Here, the role of RA is not a treatment in itself; it is just an adjunctive therapy to achieve optimal stent expansion. In addition, lesion selection bias of candidates for the RA technique may be unavoidable; a large sample size will be required to prove the clinical impact of RA.
Conclusions
OCT-guided RA was able to obtain larger stent expansion compared with IVUS-guided RA. To decide the need for burr upsizing and the final burr size, taking into account detailed calcium characteristics is an effective modification strategy when using RA. We consider that OCT-guided RA may be ideal for improving the clinical outcomes of calcified coronary lesions that remain challenging.
|
Impact on daily practice Burr upsizing was more frequently performed and the final burr size was larger in the OCT-guided RA group compared to the IVUS-guided RA group. OCT-guided RA was associated with better stent expansion, and may be ideal for improving the clinical outcomes of calcified coronary lesions. |
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

