Abstract
The interest in rotational atherectomy (RA) has increased over the past decade as a consequence of more complex and calcified coronary stenoses being attempted with percutaneous coronary interventions. Yet adoption of RA is hampered by several factors: amongst others, by the lack of a standardised protocol. This European expert consensus document stems from the awareness of the large heterogeneity in the protocols adopted to perform rotational atherectomy. The objective of the present document is to provide some points of consensus among highly experienced operators on the most controversial steps of RA in an attempt to build the basis of a standardised and universally accepted protocol.
Introduction
This consensus document is the result of several discussions and follows the dedicated meeting of highly experienced European rotablator expert operators which took place in Paris on October 17th, 2013. The objective of this meeting was to reflect upon the role of rotational atherectomy in contemporary percutaneous coronary revascularisations (PCI). Initial interest in its use dwindled following trials showing high restenosis rates1-3, but it has undergone a resurgence of interest (Figure 1) mainly due to:
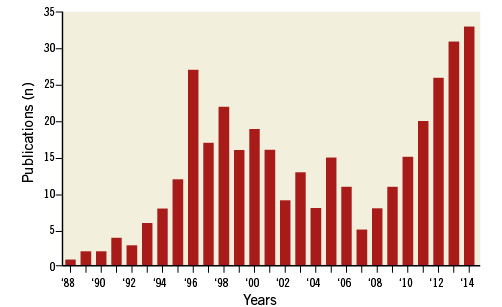
Figure 1. Number of publications over the years on coronary rotational atherectomy (source Pubmed).
a) Ageing of the population, with the more frequent referral to cardiac catheterisation of elderly patients with heavily calcified coronary arteries;
b) Expansion of PCI indications to more challenging anatomic settings as a consequence of the improved restenosis rate following drug-eluting stents.
Despite the development of more supportive catheters, very low-profile balloons with higher inflation pressure facility, and alternative plaque modifying technology, the crossing of a heavily calcified lesion might require rotational atherectomy. Nevertheless, universal adoption of this technique has been hampered by:
– concern about the complexity of the rotablator procedures and potential procedure-related complications,
– lack of standardised protocols,
– lack of structured and widely available training programmes.
As a result of these factors and of the national reimbursement policies, in European countries the rate of rotational atherectomy as a function of total PCI number ranges from 0.8% to 3.1% (Table 1). The aim of the consensus meeting was to agree on a standardised protocol for the correct performance of rotational atherectomy which would serve as guidance for training programmes and in daily procedures, and to encourage training to a broader interventional audience to correct the erroneous perception of rotational atherectomy as an exclusive technique.
A standardised protocol for rotational atherectomy
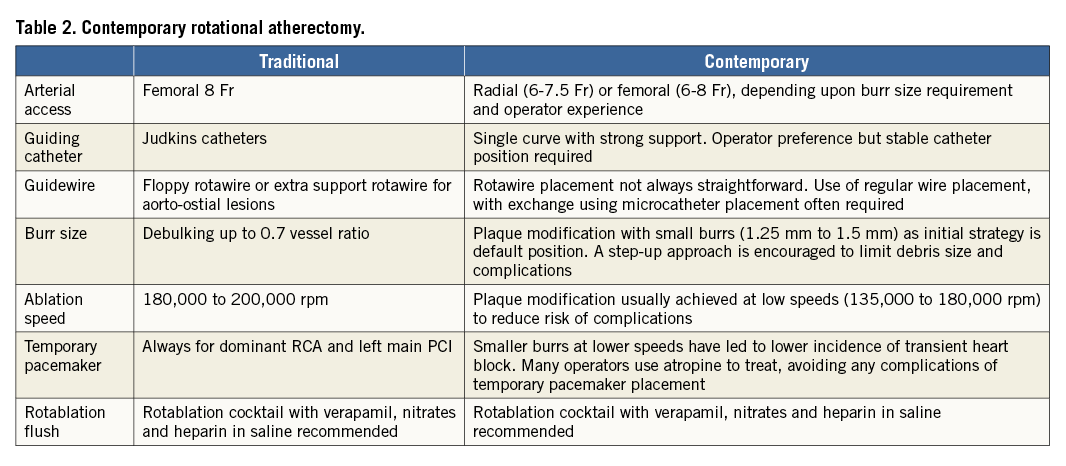
This document focuses on the key points of rotational atherectomy protocol, with particular emphasis on those characterised by heterogeneity among operators (Table 2). For a more thorough overview of the literature and technical description of the devices and techniques, the reader is referred to the PCR-EAPCI textbook4.
Main objective of rotational atherectomy
In the pre-stent era, rotational atherectomy was developed to debulk atherosclerotic plaque as an adjunct to balloon angioplasty, but disappointingly high restenosis rates limited its use. With the advent of stent technology, the adjunctive role of rotational atherectomy remained limited by a lack of impact on restenosis, if anything increasing it, by ablation injury to the vessel outside the stent1-3.
The contemporary objective of rotational atherectomy is plaque modification4,5. In most cases, the simple passage of a single burr is sufficient to smoothen the vessel lumen, or to disrupt the continuity of intravascular calcium rings, to enable subsequent balloon dilatation and stent implantation. Nevertheless, there might still be angiographic settings where a more extensive rotablation is desirable, i.e., ostial lesions, prevention of plaque shift or prolapse, which might be safely achieved with a step-up increase in burr size.
Arterial access
Rotational atherectomy can be performed during either transfemoral or transradial procedures. The smaller size of the guiding catheters routinely used during transradial procedures does not represent a limitation6, considering that plaque modification is easily achieved with a 1.25 or a 1.5 mm burr in most cases. Even in cases which require more extensive rotablation with a bigger burr size, transradial sheathless guiding catheters with an internal diameter of 7.5 Fr can be safely used.
Guiding catheter
Most rotablator procedures can be performed with a 6 Fr guiding catheter which can accommodate burrs up to 1.75 mm (though with some friction). With more extensive rotablation (i.e., with burr sizes ≥1.75 mm), 7 Fr guiding catheters or transradial sheathless guiding catheters might be necessary. The choice of the type of guiding catheter, i.e., curvature, side holes, etc., mostly depends on the vessel anatomy and on the expected back-up support needed; however, particular attention should be paid to the coaxial positioning of the guiding catheter that is required for a successful RA. Single curve guiding catheters, i.e., EBU, XB or Judkins catheters, might be associated with less friction and resistance to the burr passage in the proximity of the tip of the catheter.
Guidewire
The rotawire is notoriously fickle in its performance as a stand-alone coronary guidewire. Many operators use their regular PCI guidewire to advance through the often complex anatomy and switch to the rotawire using over-the-wire balloon or microcatheter technology. Most rotablator procedures can be performed with the floppy rotawire, while the extra-support rotawire might be particularly useful in case of aorto-ostial lesions. To prevent wire fracture or burr lodging, before starting the runs of rotablation, particular attention should be paid to position the radiopaque tip of the rotawire as distal as possible from the target coronary segment. The radiopaque tip of the rotawire, in fact, thickens up to 0.014”, while the inner lumen of the burr might only be back-loaded on the 0.009” of the rotawire shaft. Likewise, it is important to bend the rotawire tip smoothly, to avoid loops or deep positioning in small side distal branches that might increase the risk of wire fracture or vascular perforation.
Size and number of burrs
There has been a shift in the thinking about burr size as the technology has altered from a debulking tool to one that modifies plaque. This has led to a decrease in the choice of burr diameter. Generally, a small burr size should be used for very tortuous anatomy and long lesions, using only larger burr sizes for aorto-ostial lesions or in large vessels with a relatively large minimum lumen diameter where small burrs would hardly affect the plaque. Commonly, a single 1.5 mm burr represents a good compromise to tackle different lesion characteristics, achieving good plaque modification, i.e., burr-to-artery ratio of 0.6, while respecting budget constraints. Nevertheless, a step-up approach starting with a 1.25 mm burr up to 1.5 mm (often) and to 1.75 mm (seldom) burr might represent a safer approach.
Burr motion and ablation speed
With rotational atherectomy, the following predictors of procedural complications have been described: a) deceleration during rotablation (>5,000 rpm); b) motion pattern of the burr; c) speed of rotablation; d) duration of individual runs. A pecking motion of the burr, i.e., a quick push-forward/pull-back movement of the burr, is the most widely adopted motion pattern among the experts. The latter contributes to minimising the deceleration during rotablation together with a short duration of individual runs (no longer than 30 seconds). Some expert operators prefer rather slower push-forward/pull-back. A safe range of speed of rotablation is between 135,000 and 180,000 rpm. A speed lower than 135,000 rpm might be associated with burr lodging, while a speed higher than 180,000 rpm might increase platelet activation and thrombotic complications7,8. The choice of the applied speed within the recommended range follows the operator’s preference. It is important to monitor rotablation speed during the runs on the console, paying attention to changes in the rotablation sound which give practical feedback about burr deceleration.
If, after several passes of the burr, it becomes clear that the lesion cannot be crossed, downsizing of the burr is recommended. If the smallest burr will not pass, then consider a change to a more supportive, or even larger French, guiding catheter. Usually, with patience, the majority of lesions will be crossed, but occasionally one has to accept there are cases which this technology is unable to penetrate.
Temporary pacemaker
Transient AV blocks might occur during rotablation of the right coronary or dominant left circumflex artery. In this case, positioning a temporary pacemaker before rotablation might preclude complications, though it should be carefully performed. Some cases of pericardial tamponade occurring after the procedure have been caused by small and undetected right ventricular perforations that became clinically manifest after removal of the pacemaker lead, particularly if concomitant glycoprotein IIb/IIIa receptor inhibitors have been given.
Although these complications can be minimised with the placement of balloon-tipped low-profile (4-5 Fr) pacing wires, with modern rotablation techniques using small burrs at lower speeds, many experienced operators seldom use temporary pacemakers, as the incidence and clinical consequences of the transient AV blocks are minimal and can be simply treated with mechanical manoeuvres (e.g., coughing, vigorous rhythmic closure of fist, etc.) and/or pharmacologic measures (e.g., atropine).
When to stop rotational atherectomy
Rotational atherectomy should be stopped when sufficient plaque modification allows optimal balloon dilatation and stent implantation. The presence of residual dogboning of a low-pressure-inflated balloon indicates insufficient plaque modification and merits further rotablation with a larger sized burr. Caution should be used in not overinflating the balloon to overcome the dogboning image, as this might result in dissections at the edges of the lesion, without achieving full balloon expansion, and compromise additional rotablation.
Stent implantation and imaging techniques
Due to the reported higher restenosis rate with bare metal stents2, drug-eluting stents should be implanted after rotational atherectomy. Intracoronary imaging techniques might be useful during rotational atherectomy to estimate the degree of calcification, as well as to assist in the selection of stent size and length. Some rotablator experts have found computed tomography coronary angiography useful for anticipating the severity of coronary calcification, predisposing to a lower threshold for rotational atherectomy. Nevertheless, blooming artefacts might not allow clear identification of the presence of soft intimal plaque component, therefore overestimating the severity of the coronary calcification.
Pharmacology
Drugs commonly used for percutaneous revascularisation are also normally administered during rotational atherectomy. There are few data to support the routine use of glycoprotein IIb/IIIa inhibitors, which should only be considered in bail-out situations.
Rotablation flushing cocktail
Connected to a side-port of the rotablator advancer is a line for the infusion of a flushing cocktail. This infusion is important to cool the rotablator turbine and to flush the coronary circulation from debris generated during the ablation. The formerly available Rotaglide cocktail (composed of egg whites and olive oil) has been replaced due to potential allergic reactions by a saline solution with equal proportions of verapamil, nitrates, and heparin (5 mg/5 mg/5,000 U in 500 ml of saline).
Management of complications
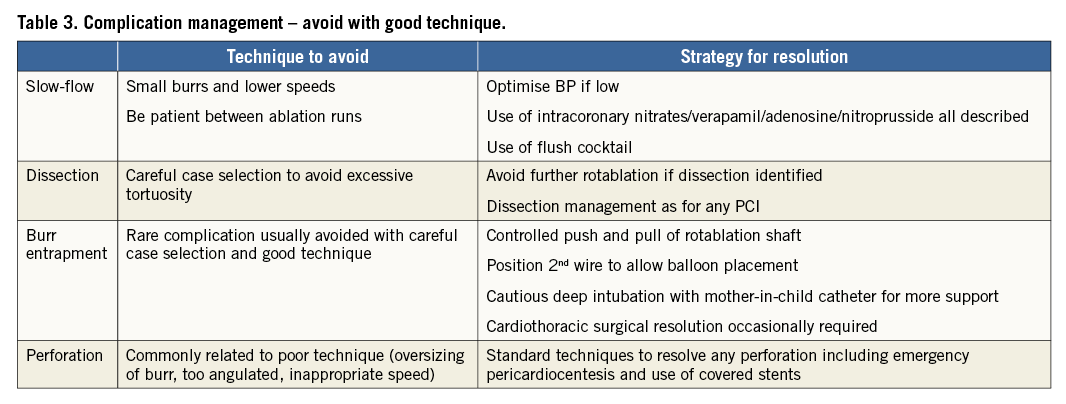
A summary of the most common complications during rotational atherectomy and relative management strategies are reported in Table 3.
Slow-flow/no-reflow
The incidence of rotablation-induced flow impairment has diminished with smaller burrs at lower speeds for shorter runs. Significant slow-flow is nearly always associated with poor technique and burr size choice. Showers of pulverised tissue take time to pass through the microvasculature, and, as long as the appropriate decisions were made regarding adequate choice of the burr size and good techniques were applied, flow impairment nearly always resolves spontaneously over time and/or might be speeded up by intracoronary nitrates. If it occurs, optimised perfusion pressure is essential, and adequate hydration of the patient is recommended.
There are users of other vasodilators to prevent and treat this phenomenon, such as verapamil, nitroprusside or adenosine, sometimes delivered directly to the microvasculature via an over-the-wire balloon or a microcatheter. Occasionally, a balloon pump can optimise perfusion pressure sufficiently to restore coronary flow.
Dissection
Once a severe dissection is identified at the control angiography, rotablation should be stopped, as further passages of the burr can aggravate the dissection plane or even cause vessel wall rupture. Management of the dissection is similar to that of conventional PCI. With suboptimal plaque modification, if the risk of vessel closure has been minimised and there is preservation of antegrade flow, the procedure could be stopped and a second attempt planned for three to four weeks later in order to allow healing of the dissection.
Burr lodged
Lodging of the burr within the stenotic segment is a serious complication which might require surgical intervention9. This complication is best prevented by safely performing the rotablation protocol (i.e., pecking motion, short duration of ablation, short segment ablated during the runs, small burr size, avoiding extreme angulation). In addition, the fusiform shape of the 1.25 mm burr can cause a sudden jump forward of the burr when activated if the stored energy has not been dispersed by moving the burr a few mm back and forth before activation. The more olive-shaped 1.5-2 mm burrs are less likely to entrap, although poor technique remains the most important element of this rare complication.
When it occurs, it is useless or even counterproductive to keep pushing on the pedal of rotablation, while the following possible strategies might help to free the burr:
a) controlled push and pull on the burr drive shaft;
b) positioning of a second wire (possibly through a second guiding catheter), eventually with a balloon inflation next to the burr, might free the device;
c) cautious deep intubation of the guiding catheter, sometimes of a mother-in-child catheter (after cutting the proximal connector of the burr drive shaft) might help to support a controlled push and pull of the drive shaft.
Perforation
To prevent perforations linked to the rotawire, particular attention should be paid to placing its tip in the distal part of the main vessel, avoiding small side branches. This position should be monitored during the procedure as the wire might be displaced easily due to the exchange of burrs and balloons. When perforation occurs, the management is similar to that of other guidewires.
Perforation linked specifically to the rotablation is uncommon, and is usually due to poor technique (oversizing of burr, “pushing” of burr rather than pecking) or poor choice of vessel (extreme angulation or small vessel size). Its management depends on the degree of perforation and cardiac compromise. It can range from a catastrophic emergency to a localised coronary injury. The use of covered stents can be lifesaving without sacrificing the disrupted vessel. Pericardial drainage might be needed. Sometimes the perforated segment cannot be treated percutaneously, in which case a balloon should be inflated proximal to the perforated segment to staunch flow whilst arrangements are made to perform urgent coronary bypass surgery.
Specific indications for rotational atherectomy
OSTIAL LESIONS
Ostial lesions are frequently heavily calcified and might be more prone to plaque shift, which is especially important in the context of bifurcations. A lower rate of acute side branch occlusion has been reported after rotational atherectomy of ostial side branch lesions before stenting of the main vessel10,11. In addition, aggressive balloon dilatation of aorto-ostial lesions might even be associated with retrograde dissections from the coronary ostium to the ascending aorta. Therefore, expert operators recommend a more extensive plaque modification in this lesion setting (i.e., debulking), in order to improve stent expansion and stent-wall apposition. In performing rotablation of aorto-ostial lesions, it is important to keep the guiding catheter coaxial in order to avoid burr lodging. A larger sized guiding catheter might be needed in order to: a) possibly start rotablation already in the guiding catheter; b) prevent the burr jumping over the ostium, therefore missing the stenosis.
UNPROTECTED LEFT MAIN STENOSIS
Rotational atherectomy has been reported as a viable option in patients with heavily calcified unprotected left main coronary stenoses10,12. Although this represents an off-label indication not currently recommended by guidelines13, it might represent the only option in patients at high surgical risk and in case of failure of the balloon to cross or to dilate the stenosis. Due to the potential haemodynamic effects of slow-flow after the burr passage, a cautious step-up approach starting with a 1.25 mm burr is recommended. Haemodynamic support should be dictated rather by the left ventricular function and by the related risk assessment of performing a PCI in an unprotected left main stenosis, rather than by the performance of the rotablation procedure.
CHRONIC TOTAL OCCLUSIONS
Rotational atherectomy might be a useful tool in chronic total occlusions (CTO) in case the guidewire has crossed the occlusion, but the balloon failed to cross or to dilate the stenosis. In this situation, the challenge is represented by the exchange of the CTO wire with the rotawire. This could be done with the use of dedicated catheters (i.e., microcatheters, Tornus® or Corsair®; both ASAHI Intecc, Aichi, Japan). After wire exchange, it is equally important to confirm the positioning of the rotawire in the distal vascular lumen (by contralateral injection). A single run with the 1.25 mm burr followed by balloon dilatation is sufficient to achieve good plaque modification in most cases.
STENT ABLATION
Rotational atherectomy has been successfully applied to cases of suboptimally expanded stents. This procedure has a high risk of burr lodging and should be discouraged. This situation should be prevented with optimal lesion preparation before stent implantation. When it occurs, rotablation of an underexpanded stent should be considered with extreme caution in highly experienced hands, preferably with surgical back-up.
WHO SHOULD DO RA?
Optimal outcomes for rotational atherectomy procedures are achieved by regular users of this technology. Therefore, it might be safer to consider transferring the patient to a high-volume centre, experienced in the rotablation procedure. This would enable the new rotablator operator to gain experience quickly while performing this complex procedure with a highly experienced team. In some countries, physicians intending to perform rotablation must:
– attend a rotablation certification course,
– complete some RA cases with a recognised RA proctor, and
– be signed off by the RA training directorate.
This is not uniform, but it emphasises that, as with all things, good training and support lead to better outcomes for the operator and the patient.
ON-SITE SURGICAL COVER
Complications of RA are uncommon in experienced hands. The nature of the device does mean, however, that occasional high-risk complications can occur, and under these circumstances the availability of immediate bypass facilities can be lifesaving. Nonetheless, the vast majority of cases performed do not require surgery, and it would seem restrictive to compel every case to be done in a surgical facility. The availability of surgical back-up during the learning curve may be a good compromise before starting a regular rotational atherectomy programme in a cathlab without an on-site surgical facility.
Many of these cases will have been discussed at a multidisciplinary forum, to decide whether RA is the appropriate strategy. As with all PCI cases, thorough documentation of procedures should be kept and a forum for audit should be available for scrutiny of individual and unit results.
Conclusion
For experienced users, contemporary rotational atherectomy offers a safe and effective means of percutaneous treatment of highly calcified obstructive coronary lesions. The technique of a smaller burr to artery ratio in a speed range between 135,000 and 180,000 rpm has led to a decrease in complications and improved outcomes. This consensus document provides a range of agreed opinion by experienced rotablation operators that can be disseminated as best practice.
Funding
The meeting of RA experts in Paris on October 17th, 2013, was facilitated and sponsored by Boston Scientific. The authors confirm that no Boston Scientific employee contributed to the preparation of the consensus document.
Conflict of interest statement
E. Barbato declares honoraria and institutional research grants from Boston Scientific to the Cardiovascular Research Center, Aalst, Belgium. G. Gaul declares teaching honoraria from Boston Scientific for rotablation workshops. A. Khashaba declares proctorship fees for rotablation from Boston Scientific. S. Sharma declares speakers bureau from Abbott, Boston Scientific, Cardiovascular Systems Inc., and TriReme Medical. I. Sjögren declares consultancy fees from Boston Scientific and AstraZeneca. G. Szabó has a contract with Boston Scientific for proctoring RA in the eastern part of Europe. W. Wijns declares institutional research grants from Boston Scientific to the Cardiovascular Research Center, Aalst, Belgium. S. Windecker declares institutional research grants from Abbott, Biotronik, Boston Scientific, Edwards Lifesciences, Medtronic, Medicines Company and St. Jude, and speaker fees from AstraZeneca, Eli Lilly, Abbott, Biotronik, Boston Scientific, Bayer and Biosensors. A. de Belder declares honoraria from Boston Scientific for the UK rotational atherectomy training programme and proctoring trainees. The other authors have no conflicts of interest to declare. EAPCI Review Co-ordinator, A. Colombo, has no conflicts of interest to declare. The document reviewers have no conflicts of interest to declare.

