Abstract
Aims: Rotational atherectomy (RA) is frequently performed to modify complex fibrocalcific coronary lesions with high procedural success. A stuck rotablator is a rare but life-threatening complication. However, its description remains sporadic and it has never been systematically analysed. The aim of this analysis is to present our experience and summarise the available literature about stuck rotablator, and to identify risk factors and possible management strategies for this complication.
Methods and results: We analysed our experience of 442 RA procedures and identified four cases of stuck rotablator. Two of these cases were rotablations in freshly implanted stents. All cases were managed percutaneously. We further analysed the available literature and identified a total of 11 reports with 14 cases of a stuck rotablator burr; seven were managed surgically and seven with endovascular approaches. Based on our experience and the literature review we developed an algorithm to guide operators while managing this complication.
Conclusions: Entrapment of a rotablation burr is a rare but very serious complication of RA. Operators performing RA should be aware of this risk and be prepared to manage it adequately. In our experience, the risk seems to be higher when rotablating freshly implanted underexpanded stents.
Introduction
Percutaneous coronary intervention (PCI) with intracoronary stents is the most common procedure for the invasive treatment of patients with coronary artery disease1. Although stent implantation is feasible in most cases, severely calcified coronary lesions pose a specific challenge as balloon dilatation and stent placement can be difficult or even impossible2,3. Rotational atherectomy (RA) has repeatedly been shown to have superior success rates when dealing with such complex fibrocalcific lesions4-8. Procedural success rates over 90% and complication rates below 5% are routinely achieved after RA in otherwise untreatable complex lesions7,8. RA has inherent risks, but severe complications such as no/slow flow, coronary perforation and shock are observed in less than 2% of the procedures4,6. One of these rare but life-threatening complications is a stuck rotablator, also known as entrapment of the rotablation burr or trapped rotablator. The event can be defined as entrapment of the rotablation burr in a coronary lesion with the impossibility to rotate or retrieve the burr. Stuck rotablator can lead to acute coronary occlusion and sometimes requires immediate cardiac surgery. The aim of this analysis is to present our experience and summarise the available literature about stuck rotablator, and to identify risk factors and possible management strategies for this life-threatening complication.
Materials and methods
In our institutional database, installed in 2003 and which has since covered 15,295 PCI procedures, 442 coronary interventions with RA (2.89%) have been documented. Entrapment of the rotablation burr which was defined as the inability to move the burr backwards without additional manoeuvres during or after rotablation of a coronary lesion was retrospectively identified. A review of angiographic and procedural details was performed in all identified cases to ensure entrapment had occurred, and all interventional manoeuvres to retract the burr were documented. Clinical follow-up was performed for all patients. Written informed consent was obtained prior to PCI, and data collection was approved by the local ethics committee.
We further analysed the available literature via a PubMed search using the keywords “rotablator”, “rotational atherectomy”, “entrapment of rotablator burr”, “stuck rotablator” and “complications of rotablation”. All articles describing this complication were analysed, and further publications were identified through the reference list of the original articles. All publications were scrutinised to identify patient demographics, diagnosis, comorbidity and procedural data (when available) in order to look at possible patient, lesion or procedure-related risk factors. In addition, all techniques used to retrieve the rotablator were identified and classified depending on the original idea and devices used.
Results
CLINICAL CASES
During a nine-year period (January 2003 to January 2012), a total of 442 rotablation procedures were performed at our institution. During this period, four patients with confirmed entrapment of a rotablator burr were identified. These cases were performed by two operators, and all patients were managed by endovascular manoeuvres without surgical intervention. In two patients coronary blood flow was initially reduced from TIMI 3 to TIMI 0-1; in both patients rotablation was performed in a freshly implanted underexpanded stent. A comparison of baseline clinical and key procedural aspects of these patients to the overall RA population is shown in Table 1. The four cases are briefly described below.
CASE 1
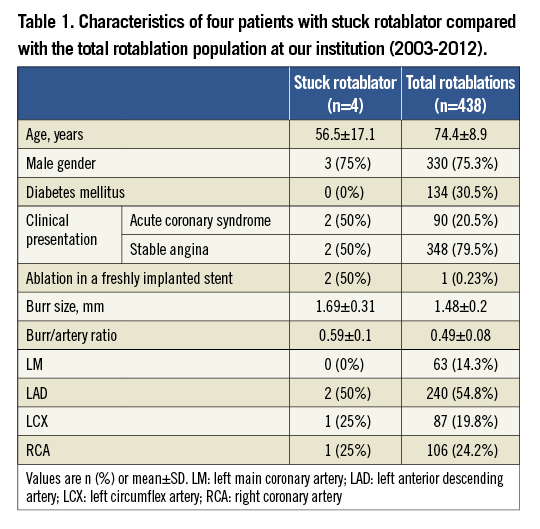
A 46-year-old gentleman was referred to our centre after an unsuccessful primary PCI for an anterolateral myocardial infarction treated with direct stenting of the proximal left anterior descending (LAD) coronary artery using a bare metal stent (BMS). The stent was not sufficiently expanded (Figure 1A). We initially postdilated the stent with non-compliant balloons at high pressures but did not achieve any further expansion and therefore decided to rotablate the stent. Using a 7 Fr guiding catheter we advanced a rotawire to the distal LAD and started rotablation with a 1.75 mm burr. Suddenly, the burr was entrapped in the stent and subsequent pullback manoeuvres were not successful. Simultaneously, the blood flow in the LAD decreased to TIMI 0 (Figure 1B). In order to disengage the burr, a second 7 Fr guiding catheter was placed through a separate sheath in the contralateral femoral artery and the entrapped burr was crossed with a polymer-coated wire (Pilot 50; Abbott Vascular, Santa Clara, CA, USA). Sequential balloon dilatation with 1.5, 2.0 and 3.0 mm balloons between the entrapped burr and the stent was performed, which finally enabled us to pull the rotablator system into the guiding catheter (Figure 1C). Despite incomplete rotablation of the distal part of the stent, it successfully expanded using a non-compliant balloon. These manoeuvres led to an extensive dissection of the left main bifurcation which was successfully treated with drug-eluting stents (V-stenting technique). The original BMS was also covered with a drug-eluting stent (DES) (Figure 1D). The patient’s clinical course was further complicated by haemodynamic instability and an assist device was temporarily needed, but he was ultimately discharged in a stable condition. Due to recurrent symptoms an angiography was performed 19 months later which revealed a good angiographic result in all treated segments but a de novo lesion in the RCA, which was successfully treated with a DES after adequate predilatation.
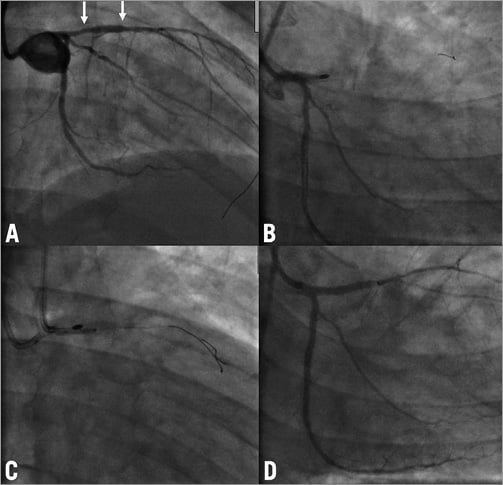
Figure 1. Case 1. A) Initial angiogram of the left coronary artery. Arrows show the proximal and distal ends of a BMS. B) A 1.75 mm burr got stuck in the stent with TIMI 0 flow distal to the burr. C) Dilation with a non-compliant balloon at the site of entrapment. D) Final result with TIMI 3 flow in LAD and LCX. BMS: bare metal stent; LAD: left anterior descending artery; LCX: left circumflex artery
CASE 2
A 38-year-old man with multiple risk factors was admitted six days after being treated with a DES in the proximal LAD because of persistent stable angina. The stent was angiographically underexpanded (Figure 2A). An attempt to perform intravascular ultrasound (IVUS) was not successful as the IVUS catheter did not cross the high-grade stenosis. Several post-dilatations of the stent with non-compliant balloons did not expand the stent. The patient was discussed with the cardiac surgeon and the decision was taken to perform stent rotablation with surgical stand-by. Using an 8 Fr guiding catheter, a rotawire was easily introduced down to the distal LAD and we started rotablation of the stent with a 1.75 mm burr at 170,000 rpm. After several cycles of rotablation the burr did not pass the stent and the very distal tip of the rotawire was fractured and approximately 1.5 cm of the wire remained in the distal LAD. The vessel had to be rewired with a new rotawire and we continued the procedure with a 2.0 mm burr. All of a sudden the burr was entrapped in the lesion with TIMI 1 flow distal to the lesion (Figure 2B). We advanced the guiding catheter deeply into the LAD towards the lesion. After this, a manual pullback with force was successful in removing both the rotablation burr and the rotawire. The LAD was then rewired with a standard floppy wire and the lesion was successfully dilated with 3.0 and 3.5 mm high pressure balloons at 30 atm. Subsequently, a sandwich stenting was performed with a further DES and IVUS documented a good result (Figure 2C and Figure 2D). Twenty-four hours after the procedure there was a slight increase in troponin T levels with no elevation in creatine kinase (CK) or CK-MB. No cardiac events have been reported during a follow-up period of three months.
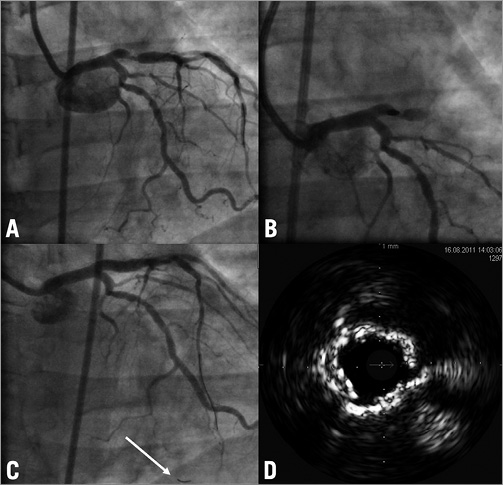
Figure 2. Case 2. A) Initial angiogram of the LAD with no visible calcification and an unexpanded stent in the proximal segment. B) Entrapment of the burr in the proximal LAD with TIMI 1 flow distal to the lesion. C) Final result of the procedure. Arrow shows a broken tip of the rotawire which remained in the coronary artery. D) IVUS image of the proximal LAD after a new DES implantation and before final post-dilatation. Note severe superficial calcification and 2 layers of stents. LAD: left anterior descending artery; IVUS: intravascular ultrasound; DES: drug-eluting stent
CASE 3
A 70-year-old man with severe three-vessel coronary artery disease (SYNTAX score = 28) presented with stable angina. He had a 90% stenosis in the proximal right coronary artery (RCA) and a 70% stenosis of the left main bifurcation (Figure 3A). Because of the patient’s severe chronic obstructive pulmonary disease the heart team’s decision was to perform PCI. After predilation of the proximal RCA we were not able to advance a stent due to severe calcification. Using a 7 Fr guiding catheter and temporary pacing we attempted to rotablate the lesion with a 1.75 mm burr. The burr passed through the stenosis but could not be retrieved from the mid part of the RCA. There was no decrease in coronary perfusion (TIMI 3) but the patient suffered from severe anginal pain. Through a second ipsilateral transfemoral access we introduced an additional 6 Fr guiding catheter, wired the distal RCA with a BMW guidewire (Abbott Vascular, Redwood City, CA, USA) and succeeded in advancing semi-compliant balloons into the lesion and sequentially dilated at the site of the stuck rotablator with 1.5, 2.0 and 3.0 mm balloons (Figure 3B). This enabled us to retrieve the rotablator with some force, resulting in an extensive dissection of the RCA (Figure 3C), which was successfully treated by implantation of four DES with a good angiographic result (Figure 3D). The further clinical course was uneventful and one week later the left main bifurcation was treated using two DES (modified T-stenting). There was no elevation of cardiac markers of necrosis and the patient was discharged in a stable condition. No cardiac events occurred during a follow-up period of three months.
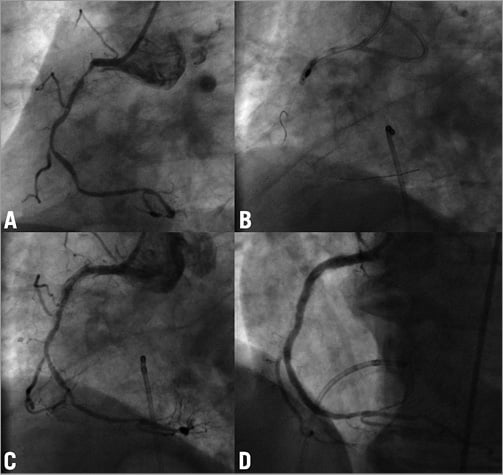
Figure 3. Case 3. A) Initial angiogram of the RCA. B) Entrapment of the burr and dilatation with a non-compliant balloon. C) Angiogram after removal of the rotablator burr resulting in severe dissection of the RCA. D) Final result of the procedure. RCA: right coronary artery
CASE 4
A 70-year-old woman with previous coronary bypass surgery was admitted with stable angina despite intensive medical treatment. Coronary angiography revealed three severely calcified tandem lesions in the native left circumflex (LCX) artery (Figure 4A) and patent grafts to the LAD and RCA. We electively decided to rotablate the native LCX. Using transfemoral access and a 7 Fr guiding catheter the rotawire was not able to pass to the distal vessel. Only a Fielder XT wire (ASAHI Intecc, Aichi, Japan) was able to cross, and the proximal lesion was easily predilated with semi-compliant balloons. However, because of severe calcification and the high-grade stenosis, the middle lesion could not be adequately dilated. We therefore exchanged for a rotawire using a microcatheter and started rotablation with a 1.25 mm burr. The proximal lesion was easily ablated without decrease of peak motion, but during ablation of the middle lesion the burr suddenly got stuck without further rotations and it was impossible to pull it back. In order to retrieve the burr we decided to cut off the rotablator system close to the advancer and removed the plastic sheath encircling the driveshaft. This made it possible to introduce a second coronary wire (Pilot 150; Abbott Vascular) through the same guiding catheter along the entrapped burr. However, no balloons were able to cross the lesion and we were only able to dilate the proximal part (Figure 4B). We then tried to intubate the guiding catheter deeply and used a GuideLiner catheter extension (Vascular Solutions Inc., Minneapolis, MN, USA) as a “mother and child” system in order to facilitate burr removal, but this was not successful. With the surgeons in the room, we were finally able to remove the burr with extreme manual force, resulting in perforation of the distal left main and dissection of both LCX and LAD with massive pericardial effusion (Figure 4C). Immediate balloon occlusion of the left main was attempted and urgent pericardiocentesis resulted in haemodynamic stabilisation. This was followed by implantation of a DES from the left main stem into the LCX which completely sealed the perforation, but the procedure was terminated with a TIMI 0 flow in the LAD and the distal LCX (Figure 4D) and an intra-aortic balloon pump was inserted. Because of haemodynamic instability we performed a second-look angiography 24 hours later and, in order to restore some antegrade flow in the LAD, a further stent was placed in the proximal segment. The further clinical course was complicated with a periprocedural myocardial infarction and the balloon pump support continued for five days. After a prolonged in-hospital stay the patient was discharged without angina and is currently in a cardiac rehabilitation programme.
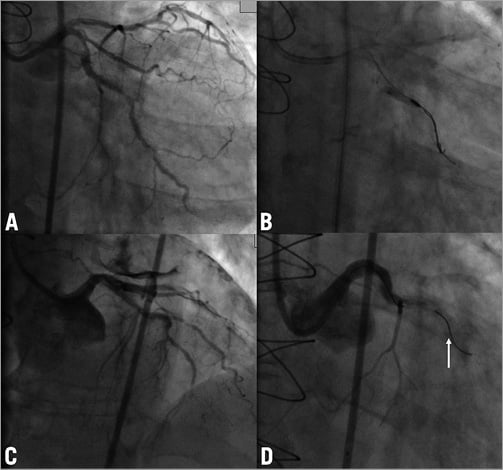
Figure 4. Case 4. A) Initial angiogram of the native LCX. B) Entrapment of the burr and dilatation with a semi-compliant balloon. C) An angiogram after removal of the burr. Note the severe pericardial effusion. D) Angiogram of the left coronary artery after sealing of the dissection and perforation with a DES; TIMI 0 flow in LCX. An arrow shows a broken tip of the rotawire. LCX: left circumflex artery; DES: drug-eluting stent
PUBLISHED EXPERIENCE
Screening the available literature we identified a total of 11 reports with 14 cases of a stuck rotablator burr9-19. In addition, Yokoi et al reported on six cases of burr entrapment during RA in an experience of 1,212 RA procedures, but a full description of these cases is not available20. The latter information supports the notion that stuck rotablator is a very rare complication. Although details of the RA procedure are not available in reports with surgical treatment of the complication, the available descriptions give important clues for the management of this life-threatening condition. Characteristics of all patients and procedures (including the four cases we are reporting) are shown in Table 2. Of the 14 patients previously reported, seven were subjected to surgical removal of the rotablator burr with subsequent bypass grafting of the target vessel9-13; one of these operations was very complex and needed resection of the pulmonary artery and reimplantation of the left coronary artery11. The surgical approach was necessary because of the inability to remove the device with interventional techniques or because of coexisting vessel perforation. The remaining cases were managed with catheter interventions. In four cases the operators were able to retrieve the burr successfully after one or serial balloon inflations at the site of entrapment14-17. Two cases were managed with a 5 Fr “mother and child” catheter or a long 5 Fr flexible straight guiding catheter inserted through the same guiding catheter and over the rotational burr shaft. These catheters were introduced through the 7 Fr guiding catheter down to the lesion and were retrieved together with the rotablation burr, avoiding extensive damage to the coronary artery19,21. To introduce a new device over the rotablator burr, operators have cut off the RA system close to the advancer (similar to the manoeuvre reported in case 4). One reported case was managed with a percutaneous snare in conjunction with partial disassembly of the rotablator device. The snare was delivered to the lesion being encircled around the burr shaft21. All these techniques aim to apply the force of retraction as close to the site of entrapment as possible.
Discussion
Coronary interventions are increasingly performed in complex calcified lesions with an incremental need for RA to facilitate balloon and stent delivery and adequate device expansion8,22,23. Moreover, RA is sometimes applied in desperate scenarios after stent implantation, such as stent underexpansion or stent jail22. RA, however, is associated with intrinsic risks and complications, which frequently lead to reluctance in its application. Entrapment of the rotablator burr is a very serious complication of RA, but it is the principal conclusion of this report that stuck rotablator is a rare phenomenon, which in most cases can be controlled with endovascular manoeuvres.
CAUSES AND RISK FACTORS FOR BURR ENTRAPMENT
The olive-like burr of the rotablator consists of two halves. The distal part of the burr is covered with diamond crumbs, but the proximal part is smooth without sputtering. Only the distal part can ablate the lesion during rotation, while the proximal part is not able to ablate while pulling back the burr, which may be a cause of entrapment in non-compliant lesions. Moreover, while moving the burr forward it is aligned to the outer curve of the vessel wall, while it is aligned to the inner curve during backward movement. In eccentric severely calcified lesions, backward movement along the inner curve can result in burr entrapment. An additional risk factor for burr entrapment - at least in our experience - may be rotablation of a freshly implanted underexpanded stent. The stent represents a hard bulky mass relatively resistant to RA both for forward and backward motion and may therefore pose a special risk. Importantly, the burr/artery ratio was noticeably high in our entrapment cases. We consciously performed stent rotablations with bigger burr sizes to provide a better contact between the burr and the stent, but this strategy appears to increase the risk of entrapment. On the other hand, it is known that RA for in-stent restenosis is relatively safe because the stent is covered with neointima.
MANAGEMENT OF STUCK ROTABLATOR
The most popular solution described in the literature was surgical removal with coronary bypass grafting. Interventional approaches can be divided into two techniques: dilatation of the lesion with balloon angioplasty, and burr removal facilitated by deep catheter intubation. In order to mobilise a stuck burr with conventional angioplasty devices, a second arterial puncture to gain additional access for a second guiding catheter would be necessary. This is due to the poor residual lumen in the guiding catheter when the rotablator is still in. To overcome this obstacle and utilise the same guiding catheter for additional devices, the rotablator system can be cut off (disassembled) distal to the advancer (including sheath, driveshaft and rotawire). After removing the sheath and leaving only the slim driveshaft surrounding the rotawire in the catheter lumen, further devices can be advanced along the rotablator remnants through the same guiding catheter15-17. Grise et al previously reported successful removal of the rotablator shaft from the LAD using this technique, but the authors abstained from continuing the interventional procedure and the patient underwent bypass surgery15. Deep intubation with subsequent pullback of all devices can be useful to focus the force on the burr and to protect the rest of the coronary artery. Once again this can be facilitated by cutting off the system and introducing a second smaller guiding or extension catheter over the drive shaft. In our second case we just used the conventional 8 Fr guiding catheter for deep intubation as there was a wide left main lumen and the lesion was proximally located in the LAD.
We are therefore proposing an algorithm for the management of a stuck rotablator which is depicted in Figure 5. A first attempt should be made interventionally with balloon dilatation at the site of the stuck burr. It can be extremely difficult to advance a “workhorse” wire distal to the lesion with a trapped burr and we suggest using a polymer-coated wire. In order to avoid a second guiding catheter, cutting the system as previously described can be performed. Another pathway is to intubate the target vessel further, either with the guiding catheter or using the “mother and child” concept. Retrieval of the burr nevertheless needs a lot of force, which should be focused on the burr by deep intubation. For this technique operators may also need to cut off the rotablator shaft. If both techniques fail the burr has to be surgically removed.
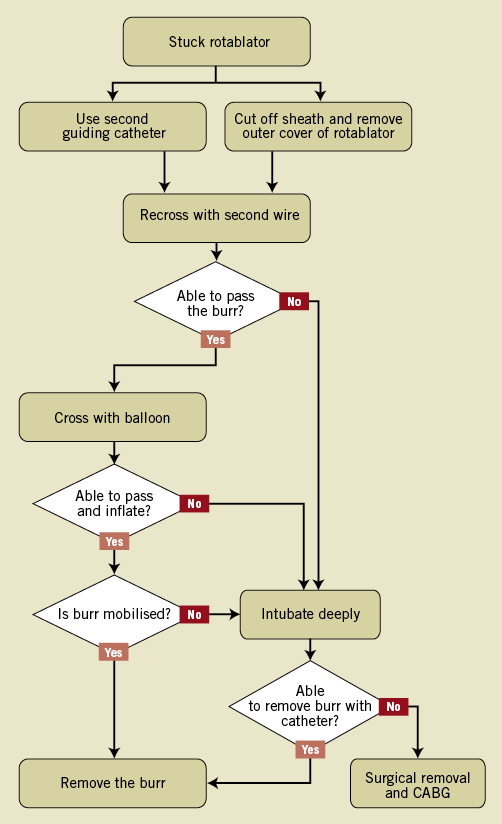
Figure 5. A step-by-step algorithm for the management of an entrapped rotablation burr. CABG: coronary artery bypass grafting
PREVENTION OF ENTRAPMENT
In aggregate, the literature indicates that stuck rotablator is a rare complication, but because of its terrible consequences operators should always be aware of it when performing RA. Entrapment of the rotablator burr has never been reported as a primary malfunction of the device or during the learning curve of less experienced operators, but rather it occurred with experienced interventionalists. Thus, mainly lesion (freshly implanted stents, eccentric calcification) and/or procedure-related factors (high burr/artery ratio) increase the risk of stuck rotablator. Even if the entrapped burr does not interrupt coronary flow, all rescue procedures have an inherent risk of complications (as in cases 1 and 4). Therefore, prevention of burr entrapment is important and is probably easier than its complicated management.
To prevent entrapment of the rotablation burr from occurring, operators should start RA in eccentric and extremely calcified lesions with relatively small burrs and a higher speed of rotation. Operators should not exert excessive forward force during burr advancement and should avoid significant decelerations of rotational speed (>5000 rpm) in order to avoid entrapment. In addition, one should also prevent the burr from jumping into the artery when the burr is activated at the coronary ostium, thereby avoiding burr entrapment in a proximal lesion.
Ho et al have previously suggested that rotablation of freshly implanted, well apposed stents is safe23, but we suggest that it must be performed very carefully using small-sized burrs and probably with higher rotational speed. Rotablation of metal stents results in early blunting of the diamonds and often several burrs of the same size will ultimately be necessary to ablate the stent. Finally, appropriate preparation of heavily calcified lesions prior to stent implantation prevents stent underexpansion and is probably the best way to avoid rotablation in freshly implanted stents.
Study limitations
One of the important limitations of this report is that a formal statistical analysis of risk factors was not feasible because of the small number of cases. Some of the published cases, especially those surgically resolved, did not provide a detailed description of the interventional procedure. In addition, operators might not feel motivated to publish complications and unsuccessful cases, which may lead to underreporting of complicated RA procedures. The presented algorithm is based on single reports without comparison of different methods. The algorithm was not tested in a broader setting, but it is accepted as our internal standard operational procedure.
Conclusions
Entrapment of a rotablation burr is a rare but very serious complication of RA which not only occurs in tortuous calcified lesions but also in relatively straight proximal coronary segments. Operators using RA should be aware of this risk and be prepared to manage it adequately. In our experience the risk seems to be higher when rotablating freshly implanted underexpanded stents. The algorithm presented is an attempt to guide operators while managing this complication.
Conflict of interest statement
M. Abdel-Wahab, G. Richardt, G. Kassner and V. Geist report supporting educational activities and receiving lecture fees from Boston Scientific. The remaining authors have no conflicts of interest to declare.

