CASE SUMMARY
BACKGROUND: A 67-year-old lady, with history of exertional angina, admitted with deterioration in her symptoms, suggestive of unstable angina.
INVESTIGATION: Physical examination, serial troponin (high sensitivity) measurement, ECG and coronary angiography.
DIAGNOSIS: Calcified mid-left anterior descending (LAD) coronary artery stenosis.
TREATMENT: Percutaneous coronary intervention (PCI) using rotational atherectomy via a right radial approach.
KEYWORDS: acute coronary syndrome, percutaneous coronary intervention (PCI), rotational atherectomy, entrapped burr, Kokesi phenomenon
Presentation of the case
A 67-year-old woman, with a background of exertional angina for five years, was admitted in our institution with symptoms suggestive of unstable angina. Her risk factors for coronary disease included hypertension and dyslipidaemia. The admission ECG showed T wave inversion in the precordial leads and ST segment depression in V5-6. Serial troponin levels were normal. Coronary angiography, performed via a right radial approach, demonstrated calcified and tortuous vessels with a critical mid-LAD stenosis. (Figure1) The LAD lesion was close to a moderate bend, and the calcification was mostly on the inner curve of that bend. There was also moderate disease in the proximal LAD, circumflex ostium and right coronary arteries. Left ventricular systolic function was normal.
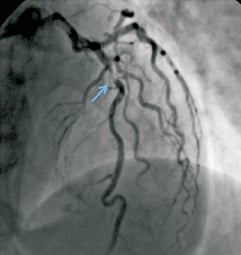
Figure 1. Left coronary angiogram showing the severe, calcified mid-LAD stenosis.
In view of the clinical presentation and calcified mid-LAD lesion, we elected to undertake PCI with rotational atherectomy prior to stent deployment. A 6 Fr XB3.5 guiding catheter (Cordis, Johnson & Johnson, Warren, NJ, USA) was used. The lesion was crossed with a0.014” Balance guidewire (Abbott Vascular, Santa Clara, CA, USA) and exchanged for a floppy RotaWire™ (Boston Scientific Corporation, Natick, MA, USA) with the help of a Finecross microcatheter (Terumo Corporation, Somerset, NJ, USA). A 1.5 mm rotational atherectomy burr was advanced to the proximity of the lesion, and rotational atherectomy at a speed of 160,000 rpm commenced. Fifteen seconds into the first run, the burr suddenly popped forward across the stenosis. Unfortunately, the burr was then trapped beyond the lesion and rotation ceased abruptly. Attempts to withdraw it only drew the guide catheter deeply into the LAD (Figure 2), and raised concerns that the guide catheter might injure the proximal coronary artery.
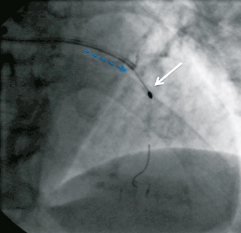
Figure 2. Attempting to withdraw the burr pulls the guide catheter deeply down the LAD artery (blue dashed arrow). A focus of calcification is evident immediately proximal to the trapped burr (white arrow).
How would I treat?
THE INVITED EXPERT’S OPINION
Burr entrapment is a recognised but rare complication of rotational atherectomy. The burr has a diamond coating for ablation on its distal surface, whilst the proximal surface of the burr is smooth: the burr can only ablate in one direction and if the burr is advanced beyond a tight calcified lesion, then it can become entrapped. This is often related to using too large a burr size, too high a burr speed or to trying to push the burr rather than pecking1.
The only ways to remove an entrapped burr are either to pull the burr out, or to send the patient for coronary surgery. In this case, pulling back the burr resulted in the guiding catheter being drawn deeply into the vessel. There are a number of other manoeuvers which can be tried, but if all are unsuccessful, then the patient will require emergency surgery. The additional difficulty in this case is the choice of a 6 Fr guiding catheter.
I would try to advance a conventional coronary guidewire beyond the burr. A stiff CTO wire may be needed to achieve this. Then a very small balloon can be used to try to dilate the lesion immediately proximal to the burr, which may create enough room for the burr to be withdrawn successfully. This may not be possible with the 6 Fr guiding catheter (inner diameter 1.75 mm): the main body of the RotaLink® shaft (Boston Scientific, Natick, MA, USA) is 1.4mm diameter, whilst most balloons have a shaft diameter of approximately 1mm, so there may not be enough room for both inside the 6Fr guiding catheter. The guiding catheter may therefore have to be exchanged for a larger diameter catheter. To so this, the RotaLink shaft needs to be cut near the hub.
This allows several options: firstly, the 6 Fr guiding catheter can be exchanged for a larger diameter guide to allow delivery of the balloon. Secondly, a goose-neck snare can be used with the noose tightened on to the burr shaft immediately proximal to the burr. Simultaneous traction on the snare and guiding catheter may successfully remove the trapped burr2. Alternatively, after the shaft has been cut, a child catheter (Heartrail® ST01; Terumo, Tokyo, Japan or GuideLiner®; Vascular Solutions, Minneapolis, MN, USA) can be advanced up to the burr: simultaneous traction on the burr shaft and counter-traction on the child catheter can result in successful retrieval of the trapped burr3,4.
Conflict of interest statement
The author has no conflict of interest to declare.
How would I treat?
THE INVITED EXPERT’S OPINION
Rotational atherectomy is proven to be effective in lesions that are poorly responsive to high balloon pressure (secondary indication), but also have to no significant value as a primary option1-3.
So the first question to address is, was rotablation indicated at all. We are often surprised to see how many severely calcified lesions actually do open at moderate balloon pressure, while some slightly calcified lesions do not break up at all. The answer to which lesions will respond to balloons and which will resist is not evident unless we try to inflate the balloon or verify the location and distribution of the superficial and deep calcium with IVUS. The deeper the calcium is located, and the more complete the calcific ring appears, the more difficulties we will encounter when only using balloons. Unfortunately these lesions often cannot be crossed with IVUS. Our policy is to only rotablate lesions with severe calcification which resisted balloon angioplasty at pressures of up to 15 bar. This occurs in about 3% of all patients. The marked eccentricity of the LAD lesion depicted in Figure1 suggests that it probably would have easily responded to a balloon.
The second point addresses the technique of rotablation. Using a1.5 mm burr in a highly calcified severely narrowed lesion that could only be crossed with the RotaWire with the assistance of aBalance and a Finecross, requires short rotation intervals of up to 5seconds, only pushing gently (the RPM speed should not drop more than 10%) in order to avoid a too large amount of debris being produced within a short time. Rotational ablation of 15 seconds without intervals to allow the debris to be washed out is too long. The burr speed was started at 160.000 rpm which is fine, but before the burr popped through the lesion, which will not occur when pushing gently, I am sure, the rpm drop exceeded 10%. It is also unclear from the presentation if nitroglycerine and verapamil were added to the continuous high pressure flushing, to avoid severe spasm and slow flow.
The situation is difficult since the burr is wedged, first, because it is not easy to get the jailed rotablator to spin again and, secondly, because it has no diamond-covered surface at the proximal part of the olive-shaped burr to allow backwards ablation. There still is agood chance to get out of this dilemma: 1) Set the rotablation speed at 200.000 rpm and try to advance the burr into the distal vessel and then try to withdraw it while spinning the burr. 2) Give nitroglycerine i.c. to release vessel tonus that is often experienced with rotablation and then proceed as in point 1. 3) Sneak a second wire (e.g., PILOT 50-200; Abbott Vascular, Santa Clara, CA, USA) parallel to the RotaWire (Boston Scientific) and burr, as well as trying to dilate the channel with a small balloon (e.g., Sprinter®; Medtronic, Minneapolis, MN, USA or Tazuna 1.25, followed by 2.0mm; Terumo, Toyko, Japan). This will sufficiently enlarge the channel.
We were successful once in a similar case when the burr jumped past an incompletely deployed stent with a very short narrow waist. A small enlargement of the trap with a balloon was sufficient to release the burr.
Conflict of interest statement
The author has no conflict of interest to declare.
How did I treat?
Actual treatment and management of case
The rotational atherectomy burr could not be withdrawn but was able to be pushed slightly further down the LAD. Angiography showed no sign of dissection and TIMI flow was normal. There was no space to advance guidewires or balloons through the 6F guide catheter, so asecond 6F XB 3.5 guide catheter was inserted via the right femoral artery. The first guide catheter was withdrawn to the ascending aorta to allow the second guide to engage the left main coronary artery (Figure3). A 0.014’’ Balance guidewire was advanced into the LAD and across the lesion, adjoining the rotational atherectomy catheter shaft. A 1.5×12mm Maverick balloon (Boston Scientific Corporation, Natick, MA, USA) crossed the lesion successfully and was inflated to 18atm (Figure4). Further inflations were performed with a 2.0mm and a 2.5×15mm NC Mercury balloon (Abbott Vascular, Santa Clara, CA, USA) to 18 atm. The burr was then easily retrieved (Figure5). A 2.5×32mm Promus Element stent (Boston Scientific Corporation, Natick, MA, USA) was then deployed at the lesion site, and post-dilated with a2.75mm balloon NC Mercury to 24 atm. A3.0×8mm Promus Element stent was used to treat the proximal LAD lesion. The angiographic result in both lesions was excellent (Figure6). The patient remained haemodynamically stable throughout the procedure. Her recovery was uncomplicated and she was discharged home on the following day.
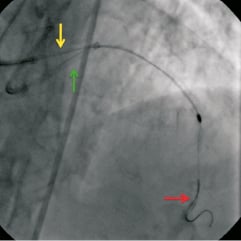
Figure 3. The first guide catheter is withdrawn into the ascending aorta (yellow arrow). A second guide catheter (green arrow) engages the left coronary ostium and a second guidewire is advanced past the burr to the distal vessel (red arrow).
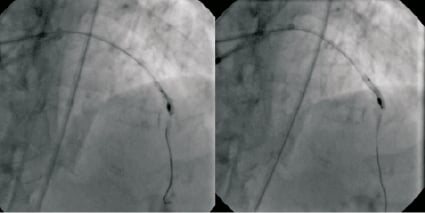
Figure 4. The calcified lesion is dilated with a 1.5 mm balloon (left panel), followed by a 2.0 mm balloon (right panel).
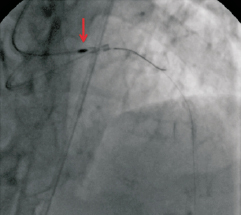
Figure 5. The burr (red arrow) is successfully retrieved into the guide catheter.
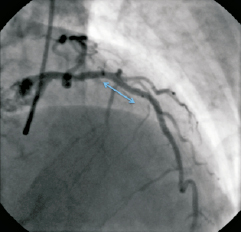
Figure 6. The final angiographic result showing the proximal LAD lesion treated with a 3.0×8 mm Promus Element stent (Boston Scientific), and the distal lesion treated with a 2.5×32 mm Promus Element stent (blue arrow), post-dilated with a 2.75 mm balloon.
Discussion
Rotational atherectomy fell out of favour in the late 1990s because restenosis rates were high when it was used as an alternative or adjunct to bare metal stenting1-3. During the last decade, the advent of drug-eluting stents has allowed more complex and extensive coronary disease to be treated, with low restenosis rates. Many of these lesions, particularly in the elderly and those with renal failure, are calcified. Since incomplete stent expansion predisposes to late stent thrombosis and to restenosis, the use of rotational atherectomy has undergone a resurgence for lesion preparation prior to stent deployment, with encouraging results4.
Entrapment of the burr is an uncommon complication of rotational coronary atherectomy. Yokoi et al5 described burr entrapment in six of 1,212 patients (0.4%) treated with rotational atherectomy over seven years in the 1990s, with only occasional case reports in the literature since then.
There appear to be two main causes of burr entrapment. The first, as occurred in this case, is the Kokesi phenomenon, named after the Japanese doll6. The doll has a head with a bulb-shaped base inserted into a slightly smaller hole in the top of the body. This mechanism of entrapment is more likely to occur when a pecking-style forward motion is used, and when a small burr is firmly pushed across a calcified narrowing at high rotational speed. The other mechanism is increasing resistance leading to burr stalling. This occurs when larger diameter burrs are used in long, heavily calcified lesions5. The likelihood of burr entrapment may be lessened by applying gentle but constant forward pressure, rather than pecking, during ablation and reducing the pressure on the burr when the rotational speed drops.
Several bailout methods to retrieve a trapped burr have been described, including using a similar approach to this case7. Hydrophilic and stiffer guidewires may be needed to cross the lesion, with a risk of downstream vessel dissection8. Balloon inflation may just stretch the more pliable portion of the vessel wall leaving the calcification intact. Another approach, which requires a7Fr guide catheter, is to cut the proximal end of the atherectomy catheter shaft and RotaWire (Boston Scientific Corporation, Natick, MA, USA), and advance asnare over the shaft down close to the lesion. Pulling on the tightened snare applies better traction to the burr than force applied to the proximal end of the catheter shaft9. This method, inspired by pacemaker lead extraction techniques, may reduce the risk of coronary dissection or avulsion. In a similar manner, a GuideLiner catheter (Vascular Solutions, Inc., Minneapolis, MN,USA) could be advanced down the coronary artery over a cut atherectomy catheter shaft to provide counter-traction close to the site of the lesion10. The distal end of the GuideLiner might also facilitate burr retrieval by favourably altering the shaft alignment close to the lesion. If none of these approaches work, surgical removal is the last, but by no means least, bailout option6.
Rotational atherectomy remains a very useful tool for treating lesions unresponsive to balloon inflation, and for debulking calcified lesions to facilitate optimal stent expansion. Interventional cardiologists performing rotational atherectomy should be aware of this rare complication and the options available for retrieving an entrapped burr.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Online data supplement
Moving images 1 and 2. Left coronary angiogram showing the severe, calcified mid-LAD stenosis.
Moving image 3. Attempting to withdraw the burr pulls the guide catheter deeply down the LAD artery. A focus of calcification is evident immediately proximal to the trapped.
Moving image 4. The burr is successfully retrieved into the guide catheter.
Moving images 5 and 6. Final angiographic result showing the proximal LAD lesion treated with a 3.0×8mm Promus Element stent, and the distal lesion treated with a 2.5×32mm Promus Element stent, post-dilated with a 2.75mm balloon.

