Case summary
Background: A 61-year-old female with a history of type-II (tablet-controlled) diabetes, obesity and dyslipidaemia presented with chest pain and dyspnoea
Investigation: Physical examination, electrocardiography, laboratory tests, coronary angiography
Diagnosis: Single vessel coronary artery disease with severe calcification of a dominant right coronary artery
Treatment: Excimer laser and rotational atherectomy
Keywords: Acute coronary syndrome, coronary artery disease, calcification, percutaneous coronary intervention, laser angioplasty, rotational atherectomy
How should I treat?
Presentation of the case
A 61-year-old female with type-II diabetes, obesity (BMI 33) and dyslipidaemia presented with chest pain and dyspnoea. An electrocardiogram (ECG) revealed transient elevation of the ST-segments in leads II, III and avF, which had normalised on arrival in the emergency department. An acute coronary syndrome was diagnosed and the patient received aspirin 300 mg, clopidogrel 600 mg, enoxaparin 1 mg/kg BD and diltiazem SR 120 mg BD (beta-blockers were contra-indicated due to a history of severe asthma). Troponin T was elevated and she underwent urgent coronary angiography via the right radial artery with 6 Fr catheters.
The left coronary system was unobstructed with minor atheroma only (Figure 1 and Movie 1).
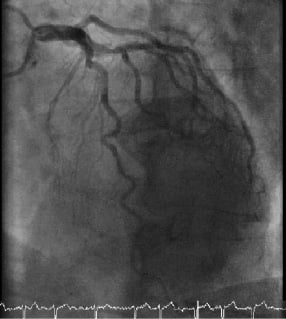
Figure 1. The left coronary artery.
A large dominant right coronary artery had a tight mid-course stenosis with a hazy appearance, suggesting either extensive calcification or thrombus (Figure 2 and Movie 2).
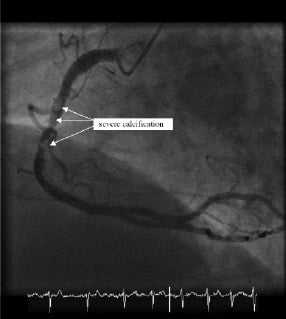
Figure 2. The right coronary artery before percutaneous coronary intervention.
Using an Amplatz Right 1 guide catheter, a Luge™ wire (Boston Scientific, Natick, MA, USA) was advanced to the distal vessel, but a 0.85 mm NIC Nano® Balloon (SIS Medical, Winterthur, Switzerland), the lowest profile balloon available in our catheter lab, would not cross the lesion, despite prolonged attempts and exchanging to a more supportive RunWay™ guide catheter (Boston Scientific, Natick, MA, USA). In this situation we would usually proceed to rotational atherectomy, but we were unable to exchange a RotaWire™ (Boston Scientific, USA) and it would not cross independently.
How could I treat?
The Invited Experts’ opinion
My strategy to crossing this calcified lesion in a tortuous artery would be to first, use a more supportive guiding catheter, such as an Amplatz Left 1. Improving guiding catheter support by deep insertion into the RCA (also known as “deep seating” or “deep intubation”) is critical for crossing complex calcified lesions1-3. Careful attention to the pressure wave form is necessary to avoid proximal RCA dissection from the guiding catheter.
Next, I would attempt to cross with a stiffer shaft guidewire such as an Ironman and consider placing a second buddy guidewire, such as a Balance Heavy Weight guidewire. I would then try the 1.25 mm Sprinter Legend (Medtronic, Minneapolis, MN, USA), which has an excellent crossing profile. If I could not fully cross the lesion, I would still inflate the balloon in the proximal part of the lesion. In some cases, the balloon is actually “stuck” on the lesion and dilating proximally may then facilitate advancing the balloon further into the lesion. If this failed, I would use a Tornus device, (ASAHI Tornus; Abbott Vascular, Santa Clara, CA, USA), which usually enables insertion of low profile balloons4. The Tornus device can even be useful when it only partially crosses the lesion5, and may also facilitate guidewire exchange for the Rotawire.
We have also recently developed a new monorail-design crossing catheter, Lev-OR™ (Baylis Medical, Montreal, Canada), which will shortly be used in human clinical trials. This hydrophilic coated catheter has a nitinol distal segment for increased push, and a tapered distal tip with inner diameter of 0.017” and outer diameter of 0.022”.
How could I treat?
The Invited Experts’ opinion
In this case, a quite “young” 61-year-old female with troponin-T positive acute coronary syndrome and transient ST-segment elevation in leads II, III and avF, had an urgent coronary angiography which revealed the culprit lesion in the dominant right coronary artery, whilst minor coronary atheroma was present in the left coronary system. The hazy appearance of the eccentric tight lesion in the mid-part of RCA suggested either extensive calcification or thrombus. Due to the fact that no impaired haemodynamic situations are described, and no flow-limiting coronary lesion in the left coronary system obliges surgical revascularisation, the patient was listed for immediate-PCI, whilst she is undoubtedly at high surgical risk, even with this difficult anatomy and consistency of lesions (calcified plus thrombotic).
Accepting, that she is a true surgical turndown, the real issue is how to manage this woman with PCI with her apparent combination of a severely calcified balloon-uncrossable lesion plus undoubtedly present thrombus in a setting of troponin-positive acute coronary syndrome. First, we have to face managing the crossing of this lesion, where a planned back-up strategy is critical. Guide catheter choice is of paramount importance in providing safe access of interventional tools to the treatment area, and I would take a 6 Fr AL-1 catheter (Medtronic, Minneapolis, MN, USA) for this RCA, which offers exquisite back-up qualities, i.e., from the radial approach.
A deeper intubation of the RCA, which is usually possible with a 5 Fr guiding catheter may offer additional support in a number of anatomical conditions. The transfemoral approach can lead to better support than the transradial approach in several cases, along with the option of larger caliber catheters with more back-up characteristics in the shaft.
Notwithstanding the detail that a 0.85 mm NIC Nano® Balloon (SIS Medical, Winterthur, Switzerland), one of the lowest profile balloons available, didn’t cross the lesion, I would try to attempt crossing the lesion with a 1.5 Apex-Push over-the-wire balloon (Boston Scientific, Natick, MA, USA) because of its firm and close-fitting tip configuration, i.e., as an OTW-balloon.
If this fails, the Tornus device (Asahi, Abbott Laboratories, Abbott Park, IL, USA) is a catheter made of eight stainless steel strands woven together to enhance flexibility and strength in exchanging wires, delivering balloons and providing support for CTO procedures. It is used after a wire has crossed a chronic occlusion, when a balloon will not cross. The Tornus is advanced into the CTO by up to 20 counter-clockwise rotations without strong back-up support once the tip is encroached. The device can make a smooth channel without dissection, allowing for passage of a low profile balloon1. In true CTO-cases, it can also be “screwed in” to an occlusion to provide excellent backup for guidewire crossing. If advanced through the entire lesion, it also offers the possibility of exchanging guidewires (i.e., RotaWire), which couldn’t cross independently at the very beginning of this intervention.
But due to the fact that this thrombus is undeniably present in this acute coronary syndrome case, I would not recommend the use of rotational atherectomy or the use of the Tornus device, because thrombus dislodgement downstream is very likely, and would be difficult to deal with.
In order to cross this balloon-uncrossable calcified thrombus laden lesion, the only option to safely treat both (e.g., thrombus plus the calcified plaque) is laser debulking. In contrast to rotational atherectomy, the use of an excimer laser does not require wire exchange, and can be used with high power (80 J/80 Hz). The 0.9 mm X-80 catheter (Spectranetics Corp., Colorado Springs, CO, USA) can cross even heavily calcified lesions creating a “pilot hole” that enables delivery balloons and stents in 93% of all balloon-crossing-failures2. At the same time pulsed-wave ultraviolet excimer laser light at 308 nm vaporises thrombus, suppresses platelet aggregation and enhances thrombolytic and GpIIb/IIIa activity3,4. Because it is safe, fast and easy applicable, we choose this approach in almost all balloon-crossing failures.
The next point is the eccentric and calcified nature of the lesion, where ballooning of the calcium will never be enough to enable adequately apposed stenting. Therefore atherectomy (FLEXI-CUT Directional Debulking System; Abbott Laboratories, Abbott Park, IL, USA; SilverHawk® Plaque Excision System; EV3 Inc., Plymouth, MN, USA) of the eccentric plaque may be the most desirable treatment to achieve the best possible morphological and long-term results, i.e., if stented subsequently5, but unfortunately this approach entails the absence of thrombus.
As a final point, the impact of subsequent versus a single session should be considered: reinstalling a stable TIMI flow with laser and balloon angioplasty first, followed by GpIIb/IIIa therapy for 12-24 h. (to get rid of all remaining thrombus), followed by effective debulking the eccentric calcified plaque by atherectomy/stenting the next day, may probably lead to the best possible morphological results.
On the contrary the single setting approach, with laser-induced pilot hole (including the vaporisation of adjacent thrombus), balloon angioplasty and finally creating a new straight RCA-lumen with a DES-stent is probably the most safe and adequate interventional treatment of this challenging case.
How did I treat?
Actual treatment and management of the case
A 0.9 mm X-80 excimer laser catheter (Spectranetics Inc., Colorado Springs, CO, USA) was advanced to the calcification over the Luge wire™. Several 10-second trains were performed at a fluence of 80 mJ/mm2 and repetition rate of 80 pulses per second, using the CVX-300 laser system in the presence of saline boluses. This modified the calcification sufficiently to permit an Apex 2.0x12 mm Maverick® balloon (Boston Scientific, Natick, MA, USA) to cross the lesion and pre-dilate to 12 atmospheres. However, no larger balloons would cross the lesion, which thus required more preparation.
A RotaWire™ was now easily passed to the distal vessel and rotational atherectomy performed using the Rotablator® rotational atherectomy system (Boston Scientific, USA) and a 1.25 mm burr to debulk the lesion (6 passes at 150,000 rpm) (Movie 3). The lesion was then pre-dilated with sequentially larger non-compliant (NC) balloons up to a 3.5x20 mm Mercury™ NC balloon (Abbott Vascular, Abbott Park, IL, USA), before deploying three overlapping Tsunami™ 3.5 mm bare-metal stents (Terumo Corp., Tokyo, Japan) distally and one 3.5 mm Pro-kinetic™ bare-metal stent (Biotronik, Berlin, Germany) proximally. A good angiographic result was achieved after post-dilatation with a 4.5x10 mm Mercury™ NC balloon to high pressures (22 atmospheres) (Figure 3 and Movie 4).
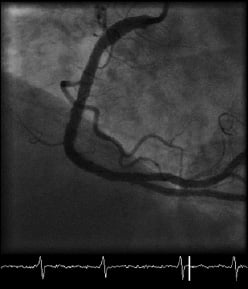
Figure 3. Final left anterior oblique view of the right coronary artery.
Discussion
Rotational atherectomy is the technique of choice for severe coronary artery calcification to facilitate adequate stent deployment1. However, it can only be utilised if a 0.009” diameter stainless steel RotaWire™ can be advanced distal to the lesion and this is not always possible. In this case, an excimer laser catheter, which only requires a standard 0.014” guidewire, was used to create a channel through the lesion. This permitted the subsequent passage of a balloon and RotaWire™ to complete the revascularisation procedure.
First generation coronary lasers proved unsuccessful, because of complications caused by thermal injury2. This has been resolved with the current pulsed wave excimer laser, combined with repeat saline boluses during lasing. Excimer laser coronary angioplasty (ELCA®) uses ultraviolet light at a wavelength of 308 nm to debulk lesions. There are three mechanisms involved in laser ablation – photochemical, photothermal and photomechanical. The photochemical process dissolves molecular bonds, using pulsed energy with a very short pulse duration (135 nanoseconds), so that the energy dissipates in between pulses. Photothermal energy from molecular vibration heats intracellular water to vaporise and rupture cells. Steam forms a vapour bubble that expands and collapses, dissolving tissue and clearing by-products away from the catheter tip – the photomechanical process. The particles generated are less than 5 µm and are absorbed by the reticuloendothelial system, as in rotablation. The tissue penetration depth of excimer lasers is low (35 to 50 µm) necessitating slow advancement of the catheter (≤0.5 mm/s) during laser emission. Removal of contrast before laser emission is mandatory, because iodinated contrast has a significant potentiating effect on the photothermal process, increasing the risk of localised thermal injury. This is avoided by clearing the manifold, line, Y connector and guide catheter using saline, and repeat boluses of saline during lasing3. The 0.9 mm and 1.4 mm laser catheters are 6 Fr compatible, whilst the 1.7 mm and 2.0 mm require a 7 Fr guide catheter.
ELCA® is currently indicated for the treatment of in-stent restenosis, long lesions, chronic total occlusions, ostial lesions, saphenous venous grafts4 and mild to moderate calcified lesions resistant to balloon inflation5. There is emerging evidence for the use of ELCA® in acute ST-segment elevation myocardial infarction to prevent distal embolisation and no-reflow in thrombus laden lesions6-8.
Conclusion
We have demonstrated that ELCA® can also be used as an adjunct to facilitate rotational atherectomy in heavily calcified lesions. We have found this combination useful in a number of similar cases and have coined the term “The Raser” to describe the amalgamation.
Online data supplement

