Case summary
Background: A 65-year-old male smoker with hypertension, type II diabetes mellitus, obstructive sleep apnoea, chronic obstructive airways disease, and hyperlipidaemia presented with acute coronary syndrome on the background of extensive coronary artery disease.
Investigation: Coronary angiography
Diagnosis: Triple vessel coronary disease
Treatment: Revascularisation
How should I treat?
Presentation of the case
A 65-year-old gentleman with a background history of uncontrolled diabetes mellitus, hypertension, hyperlipidaemia, chronic obstructive airways disease, obstructive sleep apnoea (OSA), obesity (BMI 42.0), and a current smoker developed symptoms suggestive of angina. Diagnostic coronary angiography demonstrated extensive triple vessel disease, and good left ventricular function (Figure 1, Video 1); his calculated SYNTAX score was 36, and EuroSCORE was only 2.45%. Despite this he was deemed too high risk for coronary artery bypass grafting (CABG), and subsequently referred to our institution for percutaneous coronary intervention (PCI).
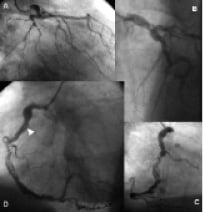
Figure 1. Diagnostic coronary angiograms (Video 1). (A) Anterior posterior caudal, and (B) left anterior oblique (LAO) cranial projection of the left coronary circulation demonstrating tortuous vessels, and significant lesions in the proximal circumflex, obtuse marginal, distal left main stem and proximal left anterior descending artery. (C) Right anterior oblique and (D) LAO projection of the right coronary artery (RCA) showing extensive calcification, tortuosity, and significant proximal, mid and distal lesions. Arrowhead indicates early appearance of filling defect seen on subsequent angiogram (Figure 2).
Before his scheduled PCI, he was admitted with acute coronary syndrome (no new ECG changes, Troponin t 0.08 µg/l, TIMI risk score 6) which resulted in transfer to our institution for urgent PCI. Repeat angiography demonstrated no new pathology in the left circulation, but a prominent filling defect was seen in the proximal right coronary artery (RCA) in addition to the series of lesions in the proximal, mid and distal vessel noted previously (Figure 2, Video 2).
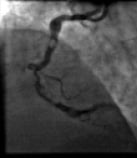
Figure 1. Diagnostic coronary angiograms (Video 1). (A) Anterior posterior caudal, and (B) left anterior oblique (LAO) cranial projection of the left coronary circulation demonstrating tortuous vessels, and significant lesions in the proximal circumflex, obtuse marginal, distal left main stem and proximal left anterior descending artery. (C) Right anterior oblique and (D) LAO projection of the right coronary artery (RCA) showing extensive calcification, tortuosity, and significant proximal, mid and distal lesions. Arrowhead indicates early appearance of filling defect seen on subsequent angiogram (Figure 2).
How could I treat?
The Invited Experts’ opinion
In this case, a 65-year-old male with significant risk factors for CAD who presents with symptoms of angina. Presumably, on the basis of a very good history of angina plus a significant number of risk factors, or after non-invasive imaging (the results of which if available, are not presented) he undergoes coronary angiography. This shows a moderate (at least) stenosis at the left main bifurcation and calcified disease throughout but particularly in the mid and distal RCA. The EuroSCORE was surprisingly low and the SYNTAX score appropriately high for this angio. He then, following surgical turndown and listing for planned- PCI, re-presents acutely.
Management: Whilst he is undoubtedly at high surgical risk, PCI in this man will not be straightforward even as a cold case and I would get consensus from more than one surgical colleague that he truly is unsuitable for coronary surgery, particularly in the light of his LMS disease. Accepting for the sake of this presentation that he is a true surgical turndown then the real issue is how do you manage, with PCI, the apparent combination of thrombus and calcium against a background of tortuous coronary artery. Having a planned, initial and backup strategy is critical. Guide catheter choice is important and I would take an 8 Fr IMA catheter for this RCA, indicating the need for a femoral approach. Alternatively to ensure good engagement in tortuous RCA the 3DRC (Medtronic, Minneapolis, MN, USA ) catheter is outstanding. Tortuous RCA can now also be accessed with the new Guideliner – a sort of tube with 1.42 mm internal diameter that fits inside the engaged standard guide to allow negotiation of a proximal shepherd’s crook tortuosity and through which a stent can be passed.
The short time from initial stable presentation to unstable re-presentation we are told is ~ 4 weeks so the new lesion (Figure 2) together with the clinical picture would suggest this is indeed what it looks like - new proximal thrombus. Routine balloon angioplasty and stenting is never going to be an option in this patient-thrombus of this size, dislodged downstream would be difficult to deal with and ballooning of the calcium will never be enough to enable adequately apposed stenting. Laser treatment to the thrombus is an option but many cathlabs do not have access to laser (including mine).
In this patient I would consider thrombus extraction as the initial strategy. Simple aspiration as per P-PCI cases (e.g., Export -Medtronic, Pronto-Pyramed) I think is probably not suitable for this patient since the thrombus is large and could be potentially be dislodged. I would use the PROXIS device which although in recent studies in P-PCI was found not to achieve the primary endpoint of complete ST resolution1 may be ideal for this case. If the thrombus cannot be extracted then this may be a problem because in such circumstances direct stenting generally with a normal stent (but perhaps with a pericardial covered stent) would be the second treatment choice for un-extractable thrombus. However the calcium requires rotablator and clearly this cannot be undertaken in the setting of a recently deployed stent. If the worse case scenario occurred and the thrombus could not be extracted, then one option would be to carefully stent and so trap the thrombus so dealing with the acute situation ensuring maximal antiplatelet therapy and then to bring the patient back for a staged rotablator in 6-8 weeks. If the thrombus can be extracted, then the distal workhorse soft wire (PT-GRAPHIX) can be balloon trapped in the guide catheter (or extended) and a micro-catheter placed antegradely over it and to the distal artery with, the workhorse wire being removed and a rota wire exchanged. Rota wires can be very difficult to negotiate through heavy calcium and exchanging out over a micro-catheter is often a simple stress free option. Rotablation with at least a 2 mm burr but probably starting with a 1.5 mm would be the basic strategy after thrombus extraction. Post rotablator non-compliant high pressure balloon will be essential to allow good lesion preparation prior to stent deployment. If he had been on DAPT prior to the second admission and thus was forming thrombus in the presence of DAPT then in the presence of a large artery, BMS might be considered an option. However, it is this sort of patient (smoker, overweight uncontrolled diabetes) who is less likely to be compliant with longer term DAPT), but, in this vessel, restenosis is likely to be high and DES is likely to result in less chance for the need to undertake repeat procedure.
Postscript: I had a similar patient but an elderly man presenting first time acutely (troponin rise) on a weekend take with less “thrombus” in an identical tortuous heavily calcified right coronary artery. I was unable to extract any “thrombus” so I undertook IVUS to see whether this was indeed thrombus and I was unable to tell because of the echo drop out form the calcium and so (safely) rotablated and stented the RCA.
How could I treat?
The Invited Experts’ opinion
This patient has clear characteristics and matches the following categories:
– left main disease with three vessels disease
– unstable angina
– good ejection fraction
– very low EuroSCORE
– very high SYNTAX score
According to the CASS study1 and the SYNTAX study2 this patient needs to be surgically revascularised; in this setting, surgical revascularisation is superior to medical therapy in terms of life expectancy and quality of life and it is superior to PCI in terms of MACE.
The presence of diabetes reinforces the surgical option. The BARI study3 demonstrated a superiority of surgery versus PCI in diabetic patients; even though the study presents very important limitations; in this study only 50% of the eligible patients were randomised and in 95% of cases the reason was the impossibility to do a PCI, but more importantly only 7.5% of randomised patients had a multivessel disease4.
In this patient the quality of coronary arteries is very however this is consistent with diabetics; the vessel calibre is small with diffuse disease. The right coronary artery is calcified with diffuse disease, the posterior descending artery is very long and probably it appears so thin because of a very low flow.
Due to the young age of the patient and the very good EF, a complete revascularisation is mandatory.
The attrition of a graft is determined by different causes5; one of the most important is the endothelial hyperplasia6. The hydrodynamic conditions are the most powerful determinant of intimal hyperplasia; a low flow with increased distal resistances is the condition to accelerate the attrition of the graft. The best working condition is when the graft has high flow, determining a high sharing stress and low systo-diastolic deformation of the wall. A graft connected to a small coronary artery with low run-off has obviously the worst working condition with low sharing stress and high wall stress.7 The only surgical technique to achieve a complete revascularisation with increased flow velocity into the graft consists of performing a sequential graft.
The sequential technique determines an increased shearing stress, insuring a longer patency of the graft even in presence of small coronary arteries.
The long-term patency of a sequential vein graft it is same of an arterial graft for the infero-lateral wall.8
According to this background, we think that the best treatment for this patient is to perform a sequential graft with a saphenous vein starting with a terminal lateral anastomoses on the posterior descending coronary artery and continuing, creating a sequential anastomoses between the vein and the postero-lateral coronary artery. The same vein will be used to create other latero-lateral anastomoses to the marginal and diagonal.
The left internal mammary artery obviously will be connected to left anterior descending coronary artery.
These surgical techniques may guarantee a complete revascularisation, treating also coronaries of one millimetre of diameter with a high probability of having a good patency rate at the medium and long term follow-up. (Figure 3)
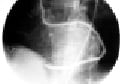
Figure 3. Sequential vein graft after 15 years in a diabetic patient. The graft is anatomised to diagonal, first marginal, second marginal and posterior descending arteries.
How did I treat?
Actual treatment and management of the case
After representing with unstable symptoms, angiography demonstrated a filling defect in the proximal RCA, which was highly suspicious of thrombus and the patient was commenced on intravenous heparin and abciximab for 12 hours. Despite anticoagulation, the filling defect did not change in appearance, raising our suspicion that it was not thrombus, but calcification. This suspicion was supported by reviewing the original angiogram that showed the presence of the defect (Figure 1D, arrow head), and his CT coronary angiogram (CTCA). This had been performed after he was scheduled for elective PCI and prior to his unstable presentation. It demonstrated extensive calcification throughout the course of the RCA, in addition to marked mid-vessel tortuosity (Figure 4). At the point corresponding to the filling defect there was severe impingement of the lumen due to extensive calcification (Figure 4B, Inset 3).
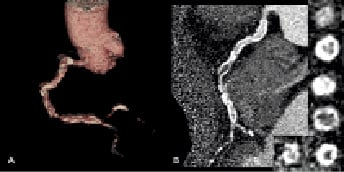
Figure 4. CT coronary angiogram (A) three dimensional (B) two dimensional multi-planar reconstruction demonstrating tortuosity and extensive calcification throughout the RCA. There is circumferential calcium with severe calcific luminal narrowing at the level where the filling defect was seen on invasive angiography (B, inset 3). The images were reconstructed using a hard kernel (B46f) to reduce the blooming effect of calcification.
The presence of circumferential calcification on CTCA was a clear indication that prior to stenting the lesion needed to be debulked with rotational atherectomy. Using a 7 Fr Amplatz guide catheter, a 0.014” soft tipped floppy wire (Pilot 50, Abbott Vascular, Santa Clara, CA, USA), we managed to cross two-thirds of the distal lesion, which was then exchanged for a 0.009” stainless steel rota wire using an over the wire (OTW) balloon. Multiple 1.25 mm burrs were performed from the proximal to the distal RCA, with the backup of a temporary pacing wire (Figure 5A, Video 3).
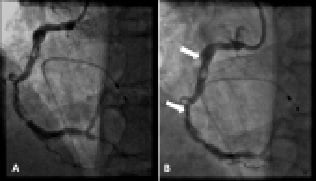
Figure 5. LAO projection of RCA indicating (A) significant improvement in appearance of proximal and distal lesions following rotational atherectomy (Video 3) (B) Most distal point reached by first stent (arrows indicate proximal and distal stent markers) after performing rotational atherectomy and balloon dilatation.
Following balloon dilatation with increasingly larger compliant balloons, we attempted to stent the distal vessel. We were unable to track a 3.5 mm by 28 mm Xience V™ stent (Abbott Vascular, Santa Clara, CA, USA) on the rota wire beyond the proximal severely angled and calcified segment, despite substantial support from the well seated 7 Fr Amplatz guide. An attempt to exchange the rota wire for a highly supportive Ironman™ wire (Abbott Vascular, Santa Clara, CA, USA) failed because the wire stiffness did not allow tracking even through an OTW balloon. The stent was finally deployed across the proximal calcified filling defect, but did not reach the more distal lesion (Figure 5B). A 5.0 mm by 12 mm Taxus™ stent (Boston Scientific, Natick, MA, USA) was deployed to cover the lesion in the proximal highly angulated segment. A further attempt to deploy a 3.5 mm by 23 mm Xience V™ into the distal lesion also failed despite the use of an additional “buddy wire” (Pilot 150) that was positioned into the distal vessel.
The Amplatz guide catheter was then exchanged for a 5 Fr Zuma™ (Medtronic, Minneapolis, MN, USA) guide catheter, which was advanced over a Pilot 50 wire, down the RCA and up to the lesion in the distal segment (Figure 6A, Video 4). Subsequently two Xience V™ stents (3.5 mm by 23 mm and 4 mm by 12 mm) were deployed without difficulty, producing an excellent angiographic result (Figure 6B, Video 4).
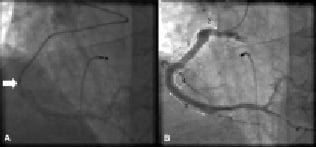
Figure 6. LAO projection of RCA showing (A) presence of a deeply intubated 5 Fr Zuma™ guide catheter (arrow). (B) Final excellent angiographic result and location of stents numbered in order of deployment: (1) Xience V™ 3.5 mm by 28 mm (2)Taxus™ 5.0 mm by 12 mm (3) Xience V™ 3.5 mm by 23 mm and (4) Xience V™ 4 mm by 12 mm (Video 4).
In view of the contrast load and the prolonged procedural time the procedure was stopped. The plan is to reassess his symptoms, and obtain evidence of reversible ischaemia by myocardial perfusion imaging before proceeding to PCI of the posterior descending artery and LMS. In the interim he has been advised to modify his risk factors.
Case discussion
This gentleman represents a high-risk patient for any type of revascularisation. However, current data from the SYNTAX trial would suggest a significantly lower MACE if he was treated by CABG.1 Nevertheless he was turned down for this in part because of his multiple uncontrolled risk factors which, at the very least, increase his risk of complication during cardiac anaesthesia. In addition, OSA is associated with a higher risk of post-operative infection such as mediastinitis, and also longer stays in the intensive care unit.2 His active cigarette consumption also increases the likelihood of graft failure.3 Post PCI, his uncontrolled risk factors increase his risk of restenosis and stent thrombosis.
Filling defects
Filling defects on coronary angiography must be interpreted within the clinical context of a patient’s presentation. Determining their exact nature is of vital importance as interventional technique can change depending on their composition, from thrombectomy and aggressive antiplatelet therapy on one hand, to rotational atherectomy on the other. In a patient presenting with unstable symptoms thrombus is the main suspect, particularly as intracoronary filling defects are the most specific angiographic marker of thrombus. These appearances however can also occur through the retention of contrast by structurally complex morphologies such as eccentric calcified plaque. A retrospective study using intravascular ultrasound (IVUS) showed that 38.5% of filling defects on angiography did not contain any thrombus,4 therefore it is important to remember that angiographic filling defects may be due to non-thrombotic pathology.
Dealing with calcified lesions
In our patient CT coronary angiography showed extensive, and in places circumferential, coronary calcification; this is essential information not only for planning a treatment strategy, but prognostically, it is predictive of future events.5 Studies comparing angiography and IVUS have shown that angiography is relatively specific (82%), but is not very sensitive (45%) for the detection of coronary calcium deposits.
This knowledge of extensive calcification helps prepare the operator for potential problems that may arise during the procedure. Examples include the potential for inadequate dilatation of the lesion due to the calcification, which results in a smaller acute luminal gain, increasing the risk of restenosis.6 As a consequence the operator may use a very high pressure balloon inflation which carries the risk of balloon rupture, coronary artery dissection or vessel rupture. Dissection occurs because the forces applied by the inflated balloon are unevenly spread between the calcified and non-calcified parts of the lesion. If it is apparent that high pressure balloon inflation is unlikely to dilate the lesion then rotational atherectomy (RA) should be considered to debulk the lesion. The operator should be aware that high inflation pressures will also be required during stent deployment for good stent strut apposition. In addition it will reduce the risk of incomplete stent expansion which can increase rates of restenosis, target lesion revascularisation (TLR) and stent thrombosis.7 Heavy calcification makes the vessel rigid and less mobile, factors which can lead to difficultly tracking equipment, as demonstrated in a subset of the TAXUS IV study where the failure rates to deliver a stent was 8.2% in calcified vessels compared to 1.8% in non-calcified vessels.8 The presence of calcium spurs can also potentially cause damage to the stent struts during attempts at stent delivery to the target lesion.
Rotational atherectomy
Rotational atherectomy (RA) relies on the principal of differential cutting where a drill bit, with an artificial diamond rotates at high speed. This selectively ablates hard lesions, especially those that are calcified, with little destruction of the surround soft tissue, and rarely arterial vessel trauma.9 RA has been shown, in lesions which could not previously be crossed or dilated to improve rates of successful balloon dilation;10 similar positive results have also been shown with regards to stenting.11 In addition RA improves arterial compliance which aides the trackability of equipment, and enables more uniform and symmetric stent deployment.12 Currently there is no evidence that RA has any benefit in terms of reduced mortality, TLR, and restenosis in either native or restenotic lesions. At present the role of RA in calcified lesions is to aid adequate lesion dilatation and facilitate stent delivery as in the present case.
Improving guide catheter support
Despite the use of RA, multiple different guidewires, and the ‘buddy wire’ technique we were still unable to deploy a stent. This is not an uncommon situation and may be due to inadequate guide catheter (GC) support which can be addressed in two different ways.13 The passive approach, which we attempted first, involves the use of a larger rigid guide catheter well seated into the ostium of the target vessel. Alternatively the active approach, which was successful in our case, involves using a smaller GC deeply intubated into the vessel. This active approach can be performed in two ways. The first uses either a 5 Fr or 6 Fr GC which is rotated and advanced into the target vessel over the guidewire to a point just proximal to the lesion; an anchor balloon can be used for additional support if required. The limitations of this technique are a risk of vessel trauma because of deep intubation, but registries have shown this to be minimal.14 Other limitations relate to the risk of air embolism, high resistance to contrast injections, ischaemia due to impaired coronary blood flow and poor vessel visualisation. It is important to ensure before embarking on this technique that the stent is 5 Fr compatible.
The second method of active support involves using the double co-axial GC technique (mother-child, or 5 in 6). There are a variety of methods but the simplest involves using a standard GC (≥6 Fr) to intubate the relevant target vessel. Within this GC a dedicated soft tipped 5 Fr catheter (Heartrail, Terumo, Tokyo, Japan) is then inserted and manipulated down the vessel as previously described. This catheter is less likely to cause trauma to the vessel because it has a straight tip (as it does not need to intubate the coronary artery), which is considerably more flexible and softer than a standard 5 Fr GC.15 Once the catheter is proximal to the lesion, stent deployment can be attempted again with the additional support provided by the 5 Fr catheter as demonstrated in our case.
Conclusion
This case reiterates that not all filling defects on angiography are intracoronary thrombus. In addition it emphasises the benefits of additional non invasive imaging, such as cardiac CT, in patients with complex coronary artery disease, to help guide an appropriate management strategy. Finally, it highlights the problems associated with tortuous calcified coronary arteries, and describes some techniques to help overcome these and potentially achieve a successful angioplasty result.
Online data supplement
Video 1. Diagnostic coronary angiograms.
Video 2. LAO projection of RCA indicating extensive proximal filling defect, and distal calcified lesion.
Video 3. LAO projection of RCA.
Video 4. Final LAO projection of RCA.
Supplementary data
To read the full content of this article, please download the PDF.
Video 1. Diagnostic coronary angiograms.
Video 2. LAO projection of RCA indicating extensive proximal filling defect, and distal calcified lesion.
Video 3. LAO projection of RCA.
Video 4. Final LAO projection of RCA.

