CASE SUMMARY
Background: A 42-year-old diabetic male suffered an uncomplicated non-ST elevation myocardial infarction and was referred for urgent coronary angiography.
Investigation: Physical examination, laboratory investigations, ECG, transthoracic echocardiogram and coronary angiography.
Diagnosis: Triple vessel disease with moderately impaired left ventricular function.
Treatment: Coronary angiography, antiplatelet and antithrombotic therapy, percutaneous intervention (PCI), intravascular ultrasound (IVUS), beta blockers, statin and angiotensin- converting enzyme inhibitor (ACEI).
Keywords: myocardial infarction, percutaneous intervention, stent fracture, mural thrombus, coronary perforation.
Presentation of the case
A 42-year-old male with diabetes and a strong family history of ischaemic heart disease suffered a non-ST elevation myocardial infarction (NSTEMI), characterised by an elevated troponin T and inferior ischaemic ECG changes. Renal function and haematology work-up was unremarkable. His medical treatment comprised aspirin 75 mg daily post loading with 300 mg, clopidogrel 75 mg daily post loading with 600mg, beta blocker, nitrates, statin and an ACEI. A resting transthoracic echocardiogram demonstrated moderately impaired left ventricular function (LVEF 40%) with an inferior region wall motion abnormality. Diagnostic coronary angiography revealed calcified triple vessel disease. The left main (LM) had a distal 60% lesion and the left anterior descending artery (LAD) showed diffuse disease; proximally 60%, mid 50% and distally 80% (Figure 1). The left circumflex artery had a mid chronic total occlusion with collaterals from the distal right coronary artery (RCA) and distal LAD. The first marginal (OM1) had a proximal 80% stenosis. The RCA had luminal irregularities with a significant stenosis of 95% at the mid segment. The SYNTAX score was calculated at 41.5. Coronary artery bypass grafting was discussed as the preferred revascularisation strategy but the patient declined surgical intervention. He underwent successful percutaneous intervention to the high grade culprit lesion in the mid RCA with a 2.5×15 mm Vision® stent (Abbott Vascular, Redwood City, CA, USA). His recovery was uncomplicated. A staged procedure to the LAD and left circumflex artery (LCx) was arranged four weeks later. The strategy for the remaining two vessels was balloon angioplasty only for the OM1 lesion, and to use a drug-eluting balloon for the distal LAD and drug-eluting stents from the mid LAD back into the LM.
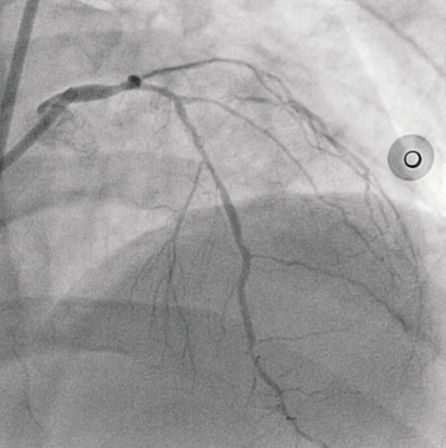
Figure 1. AP view of the left coronary system, demonstrating significant LM and LAD disease.
Access for the staged procedure was via the right femoral artery with a 7 Fr arterial sheath and EBU 3.0 guiding catheter (Medtronic Vascular, Minneapolis, MN, USA). Seven thousand units of intra-coronary heparin were administered. Intravascular ultrasound (IVUS) was used to assess the calibre and extent of disease in the LM and LAD. The luminal area of mid to distal LAD was 2.5 mm² (reference diameter 2.5 mm), the proximal LAD 4 mm² (reference diameter 3.2 mm) and the LM 4.5 mm² (reference diameter 4.1 mm). The OM1 lesion was treated first, as planned with a 2.0×15 mm Sprinter® Legend balloon (Medtronic, Minneapolis, MN, USA) inflated to 14 atm. There was a dissection noted in the mid OM1 but with anterograde TIMI 3 flow this was left alone as the vessel was deemed too small for bail-out stenting. The distal LAD was treated with a 2.25×15 mm Dior® drug-eluting balloon (Eurocor GmbH, Bonn, Germany) at 12 atm for 40seconds with an acceptable angiographic result. A drug-eluting stent, 2.5×38 mm Xience Prime® (Abbott Vascular) was subsequently deployed at 14 atms to the mid LAD, yielding a good angiographic result. The proximal LAD was stented back into the left main, using another 3.5×18 mm Xience Prime® at 10 atms (Figure 2A). There was a “waist” noted at the mid part of the stent and a non-compliant 4.0×15 mm Sprinter Legend® balloon (Medtronic) was inflated to 22 atms (Figure 2B). This resulted in extravasation of contrast at the proximal LAD, suggesting a contained mural perforation (Figure 3). Clinically the patient remained asymptomatic, and there was no haemodynamic compromise or ECG changes. Angiography with Stent Boost® (Philips Medical Systems, Eindhoven, The Netherlands) and IVUS confirmed that there was a stent strut fracture (Figure 4).
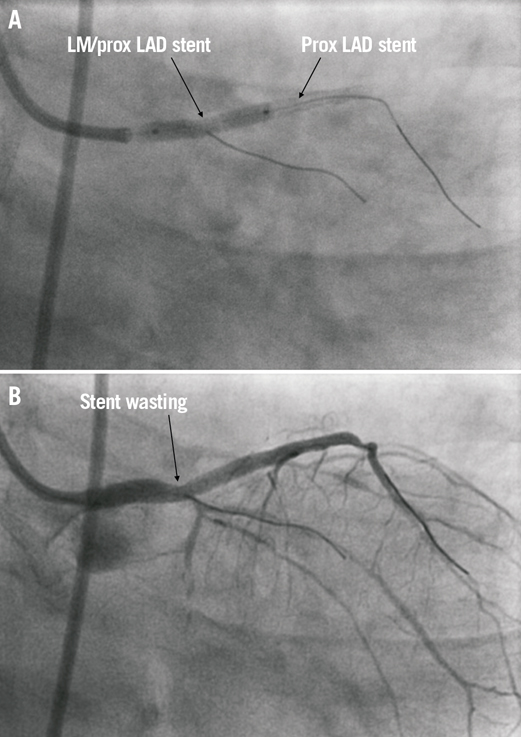
Figure 2. RAO cranial views showing: A) overlap of stents in left main and proximal LAD; and B) “waisting” of the LMS/proximal LAD post stent deployment.
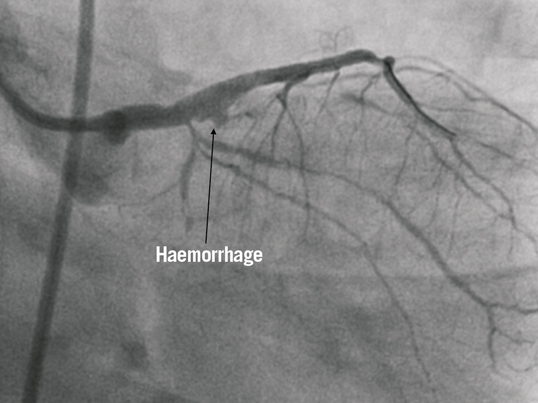
Figure 3. Extravasation of contrast suggestive of Type 1 mural perforation in the proximal LAD after post-dilation to optimise stent appearance.
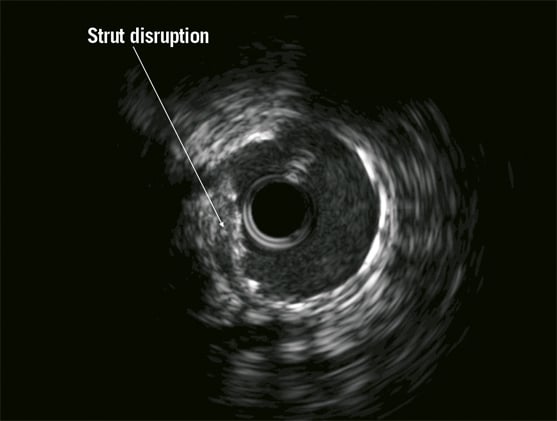
Figure 4. Intravascular ultrasound (IVUS) image demonstrating stent strut disruption and mural haemorrhage beneath the stent.
How could I treat?
THE INVITED EXPERT’S OPINION
Stent fracture is a well-known risk factor for in-stent restenosis after drug-eluting stent (DES) implantations1,2. In addition, it is rarely but potentially associated with serious adverse events such as stent thrombosis and aneurismal formation. However, there have been a few reports which discuss the risk of coronary perforation due to stent fractures. Harish et al reported a patient with a stent fracture-related coronary aneurysm. A significant stenosis in the LAD was treated with a DES. Coronary aneurysm at the DES fracture site was found two years after the initial procedure3; no adverse cardiac events occured within the first two years. Choi et al presented the coronary aneurysm at mid RCA due to stent fracture. A coronary aneurysm developed to 4 cm in diameter with cardiac tamponade occuring at three months after the DES implantation4. This patient underwent emergent cardiac surgery. Hoshi et al showed a patient with a pseudoaneurysm at the RCA ostium due to stent fracture at three months after DES implantation5. The patient suddenly developed cardiopulmonary arrest and died nine days after the detection of the pseudo coronary aneurysm by coronary CT.
In the present case, a Xience Prime stent was implanted from the left main trunk to the LAD using the single-stent crossover technique. After a high pressure post-dilatation, mural perforation occurred at the LAD ostium concomitant with the stent fracture. Perforation was classified into Ellis Type II (myocardial blush without contrast jet extravasation)6; haemodynamics were stable and the patient had no symptoms.
In my opinion, the first approach is the administration of protamine to partially reverse the effect of systemic heparinisation and prolonged balloon inflation. If the bleeding space is reduced, this patient should be conservatively managed. If bleeding increases, there might be three options. The first option is the use of a covered stent. The procedure does not seem to be difficult. However, the bleeding cavity exists just distal to the LCx ostium. This approach is associated with a high risk of LCx occlusion. The patient had impaired LV function with inferior OMI, therefore, this approach is not the best approach. The second is the surgical approach. However, the bleeding site is in a difficult position to repair the perforation. Furthermore, the stent had been already implanted from the left main trunk (LMT) to the mid LAD. The ligation of LMT and LAD and bypass grafting to LAD and LCx are necessary. The risks of this emergent surgery seem to be high. The third option is to manage the patient conservatively in the coronary care unit while diligently checking for signs of cardiac tamponade by cardiac echocardiography for 48-72 hours. Coronary CT will be performed to check the size of the pseudoaneurysm after discharge. We would recommend this approach. The site of the previously-reported ruptured aneurysm due to stent fracture was the RCA. The current case is the mural site of LAD ostium. The risk of tamponade seems to be low6,7. Shimony et al evaluated the risk of tamponade and mortality in patients with coronary perforation in a meta-analysis. The risk of tamponade was 3.3% and mortality was 0.4% in patients with an Ellis class II coronary artery perforation8. If the haemodynamics have deteriorated due to cardiac tamponade, immediate pericardiocentesis and invasive approaches, including implantation of a covered stent or emergent surgery, have to be performed after discussing the risk of these approaches with the surgeon and the patient.
Conflict of interest statement
The author has no conflicts of interest to declare.
How could I treat?
THE INVITED EXPERTS’ OPINION
Coronary artery perforation (CAP) remains a dreaded complication of percutaneous coronary intervention (PCI). A recent meta-analysis included 16 studies of 197,061 PCI procedures and identified a pooled incidence of CAP of 0.43%1. Treatment of complex lesions and use of atheroablative devices emerged as the most reproducible risk factors. In a hierarchical Bayesian random-effects model, the pooled tamponade rates were 0.4%, 3.3% and 45.7% for patients with Ellis class I, II, and III CAP, respectively. Pooled mortality rates were 0.3%, 0.4%, and 21.2% for patients with Ellis class I, II, and III CAP respectively1. Management strategies for CAP varied from observation, heparin reversal, prolonged balloon inflation, covered stent implantation, pericardiocentesis, and surgery.
Kohli et al report a case of type I mural haematoma that occurred after stent implantation in a proximal left anterior descending artery (LAD) lesion. The authors correctly performed intravascular ultrasound (IVUS) to achieve a diagnosis of what angiographically appeared as contrast extravasation. They report the presence of stent fracture.
An intramural haematoma after PCI is defined as an accumulation of blood within the medial space displacing the internal elastic membrane (IEM) inward and the external elastic membrane (EEM) outward, with or without identifiable entry and exit in contrast and usually presents as a blood-filled space with a homogeneous appearance of a relatively echo-bright pattern. A retrospective study reported IVUS identified intramural haematomas in 6.7% of PCI2. Short-term complications (non–Q-wave [myocardial infarction] MI and need for repeat revascularisation within one month) occurred mainly in the non-stented group, and long-term outcomes (death and need for repeat revascularisation at one year), occurred mainly in the stented group.
An extramural haematoma is defined as an accumulation of blood outside the arterial wall in the adventitia tissue and presents with an echo-dim pattern due to the dilution of the red blood cells and dissemination throughout an echogenic adventitia3.
In the present case, angiography documented Ellis class I CAP. Looking at the IVUS cross-sectional image, we suspect the presence of extramural haematoma. There is doubt that stent strut fracture is present (arrow, Figure 4). Rather, it seems that the stent is under-expanded (arrow) and that the haematoma leads to a compression effect at the level of stent under-expansion. No clear evidence of thrombus is present, but cannot be ruled out. Minimal luminal area appears preserved. The site of entry of the haematoma could be distal or proximal to this cross-section, and might have been created by predilatation of the lesion, stent implantation or, more likely, in the post-dilatation phase using the 4.0mm non-compliant balloon at high pressure in the proximal LAD with an IVUS reference vessel diameter of 3.2 mm. Optical coherence tomography could also be useful to delineate anatomy and diagnose stent fracture4.
We would quickly rule out the presence of pericardial effusion by echocardiography. Management options, however, remain controversial in this setting. Considering the site of the “extramural haematoma”, i.e., proximal LAD, it is mandatory to preserve adequate blood flow due to the presence of a large myocardial territory at risk of ischaemia. Extramural haematoma may evolve to more advanced CAP with a risk of pericardial effusion and eventually tamponade or may limit flow. In the present case of optimal coronary flow and clinical and haemodynamic stability, we would follow a conservative strategy of continuous monitoring in the catheterisation lab and subsequently in the intensive cardiac care unit for between 24 to 48 hours, with serial echocardiographic assessment. Periprocedural and serial troponins and/or CK-MB would be assessed to monitor myocardial damage.
In the absence of myocardial contrast staining, we would not proceed with heparin reversal and/or balloon inflation to occlude flow. Given the adequate minimal luminal area, despite the asymmetric stent expansion, we would not attempt further stent post-dilation to avoid the risk of frank CAP. We would continue with dual antiplatelet therapy. We would monitor the patient closely at follow-up with planned non-invasive imaging tests of myocardial ischaemia, such as stress-induced myocardial nuclear scans or cardiac magnetic resonance at six months, even in the absence of symptoms. Finally, and as an aside, in treating this high-risk (SYNTAX score 41.5) diabetic patient percutaneously, we may have considered treating the right coronary artery with a drug-eluting stent instead.
Conflict of interest statement
The authors have no conflicts of interest to declare.
How did I treat?
Actual treatment and management of case
Repeat angiography of the left coronary system was performed 15 minutes later demonstrating no pericardial leak or residual staining hence confirming a contained mural perforation. The patient remained stable without decompensation of vital signs. The case was discussed and reviewed by senior colleagues, and was decided for conservative management. In view of the patient’s pre-procedural use of aspirin, clopidogrel and concomitant intra-coronary heparin dose of 7,000 units during the PCI, he was given Protamine (50mg) intravenously to partially reverse the heparin. The patient was transferred to the coronary care unit for monitoring. An urgent echocardiogram was performed and showed no pericardial effusion. On review of his medication the clopidogrel was stopped and replaced with Prasugrel and the patient continued with aspirin. He was advised to continue on long-term dual antiplatelet therapy, and was discharged 48 hours later. On subsequent review, four and then twelve weeks later he was well and remained asymptomatic.
Case conclusion
This case highlights the well-documented complication of stent fracture occurring during deployment in percutaneous coronary intervention, especially in calcified arteries. Although this phenomenon is uncommon, it has been increasingly reported since the introduction of drug-eluting stents. On review of this case: in an attempt to optimise stent deployment using a non-compliant balloon, we failed to appreciate the length of the balloon at the region of the overlap of the two drug-eluting stents where we inflated at high pressures. We believe it was this overexpansion that was the mechanism that caused the acute stent fracture. In-stent restenosis at the site of stent fracture months after stent implantation has been reported frequently in the literature and the management is usually to cover the fracture site with another stent. However, stent fracture at time of implantation is uncommon and optimal management in an acute setting still remains uncertain.
Coronary stent fractures: a review of the literature
Background
Coronary stent technology has evolved prolifically since their initial usage for percutaneous revascularisation. Current generation drug-eluting stents (DES) are characterised by progressive improvement in cell architecture and alloy composition, which allows thinner stent struts, thereby facilitating deliverability, and improved visibility without compromise to the radial force1. Anecdotal reports have documented stent strut fractures manifesting as a late complication of coronary stenting, particularly affecting the first generation sirolimus-eluting stents2. Stent fractures appear to increase the risk of late restenosis and possible late or very late stent thrombosis3. This review appraises the current understanding of coronary stent fractures, their diagnosis, implications and management.
Definition
The definition of a coronary stent fracture has evolved over the last decade from merely a descriptive clinical scenario of an abnormal stent appearance during angiographic follow-up to a more precise visual inspection and description of the stent struts with intravascular imaging. A stent fracture is described as a disruption of the normal stent architecture that may occur as a complete breakage of a single strut or multiple struts and/or displacement of unconnected stent segments3.
Classification of coronary stent fracture
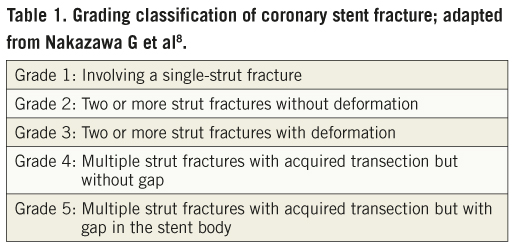
There are several classification schemes for coronary stent fractures4-9, with some overlaps between the definitions. The two most comprehensive classifications of stent fractures describe practically the morphological appearance of stent structure and help our understanding of this entity. The first is from a Korean group (Figure 5A)5 who used coronary angiography and intravascular ultrasound (IVUS) to describe the stent abnormalities in terms of the separation site of struts and the presence or absence of displacement. Three types of stent fracture are described in this classification: a) Disruption: both inner and outer struts of an angled stent are separated without displacement on the coronary angiogram. In this case the linear or curvilinear alignment of the stent is maintained; b) Avulsion: the connection of the inner struts of the angled stent is maintained with the outer strut separated and not covered with stent struts (Figure 5B); c) Displacement: the proximal and distal parts of the fractured stent struts are completely separated and displaced and thus, the linear or curvilinear alignment of the stent is not maintained. The second classification is by an American group (Table 1)8. This was based on a pathological study of heart autopsies with drug-eluting stents. They used high resolution radiographs and light microscopy to determine the classification of stent fractures. This group defines a strut fracture as a complete transection with the classification of a stent fracture being graded 1 to 5. Grade 1 involves a single strut only, grade 2 involves two or more strut fractures without deformation, grade 3 involves two or more strut fractures with deformation, grade 4 involves multiple strut fractures with acquired transection but without a gap, and grade 5 involves multiple strut fractures with acquired transection with a gap in the body of the stent. Although this is a pathological definition and not used in vivo, it has added greatly to our understanding of the mechanics of stent fracture.
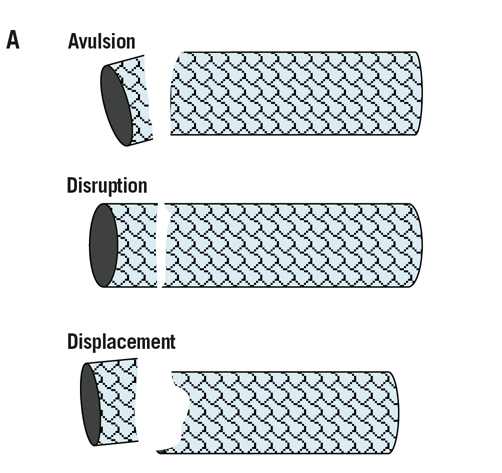
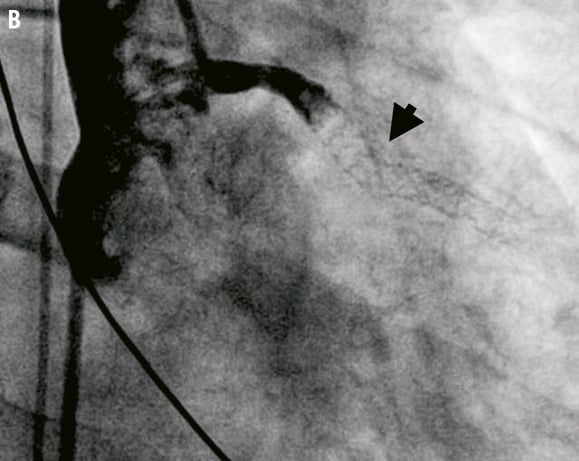
Figure 5. A. Classification of coronary stent fracture; adapted from Chung WS et al5. B. Late stent thrombosis, secondary to an avulsion fracture (arrowhead) affecting the mid segment of a sirolimus-eluting stent, deployed four years prior to this episode within the proximal LAD.
Incidence
The true incidence of coronary stent fracture is unknown, compounded by variation in the definitions used by different reports. Analysis has been retrospective mainly from case studies, mini-series and meta-analyses3,10. There are no multicentre randomised control trials to compare the incidence of stent fracture between different stent designs. For drug-eluting stents (DES), the frequency of stent fractures has been estimated at up to 4%3,9. For bare metal stents (BMS) there have only been case reports, implying that DES appear to be more susceptible to fracture. One study reported a 7.7% incidence of fracture for sirolimus-eluting stents11. This high incidence was attributable to use of long-term follow-up data using angiography and IVUS monitoring for fractures. Hence they believe other studies are likely to underestimate the incidence of stent fractures. This group used a practical and clinical definition of stent fracture: complete separation of stent segments (complete fracture) or single or multiple stent strut fracture (partial fracture) assessed by coronary angiography, plain fluoroscopy (at 30 frames/sec) and IVUS. Some investigators only describe stent fractures as complete separation of stent segments and fail to recognise partial fractures, hence decreasing the incidental yield of patients with stent fracture. The second study was an autopsy registry and reported the incidence of stent fracture to be as high as 29%8. This study examined stent fractures in sirolimus- and paclitaxel-eluting stents. The authors justify this striking figure with the use of high-definition microscopy allowing detailed examination of stent structure. Using their anatomical classification, fractures grades 1 to 4 were not shown to have any significant impact on clinical outcome. However grade 5 fractures (similar definition to other studies) resulted in a more acceptable incidence of 5.1%. Interestingly it was only the grade 5 fractures that had resulted in significant clinical outcomes (i.e., restenosis and thrombosis [67%]); grades 1 to 4 are described to be subclinical and not related to adverse outcome. However, as this was a post-mortem registry, the incidence of clinical events was highly biased. The use of IVUS and high definition microscopy imaging are the most sensitive tools for the diagnosis of stent fractures. However, as these methods are not used regularly in routine practice, the incidence of stent fractures is probably underestimated.
Natural history
The natural history of coronary stent fractures is still poorly understood. Disruption of the normal mechanical stent structure has been shown to be associated with in-stent restenosis (ISR) and late stent thrombosis, resulting in target lesion revascularisation (TLR)4,6,12. By virtue of their design, DES abrogate the incidence of ISR by inhibiting neointimal cell proliferation. However with the mechanical disruption of the stent struts interfering with the integrity of the design of the drug delivery system, exposed stent struts are not protected from the normal pathobiology of neointimal hyperplasia. Therefore the site of the strut exposure acts as a nidus for the formation of restenosis. IVUS has provided some insight into the natural history of stent fracture11, describing two scenarios: Type 1, where there is no association with coronary aneurysm; and Type 2, where there is an association with aneurysm formation. With Type 1, the fracture presents early and may be partial or complete. The fracture is related to mechanical stress forces at the hinge of the stent struts. Increased stent length and the presence of calcium magnify the risk. Type 2 presents late and is mainly complete. For instance, stenting of an aneurysmal segment or an aneurysmal reaction to the stent results in positive vessel remodelling leading to stent malapposition which causes the stent to kink resulting in the fracture. There exists a complex relationship between the stent strut structure, polymer and the eluting drug. Inflammatory reactions have been proposed to have a role in stent failure. Histology from a stent fracture in a coronary aneurysm demonstrated a predominantly lymphocytic infiltrate in the absence of giant cells suggestive of a local hypersensitivity reaction mediating formation of the aneurysm13. It is the combination of stent fracture, lack of drug elution, with the formation of coronary aneurysms and hypersensitivity reactions, that all play a part in stent fracture leading to ISR.
Both early and (very) late stent thrombosis has also been associated with stent fractures6,14. The incidence of stent thrombosis after DES implantation is approximately 1.3% per year with reported fatality rates ranging from 45%15. Exposure of stent strut piercing the coronary vessel from the luminal surface may cause injury to the vessel wall and trigger platelet activation resulting in stent thrombosis if there is no appropriate platelet inhibition with medications. Malapposition of a stent within an aneurysm may stimulate fibrin and platelet deposition. There are limited studies which have addressed the mechanism of stent thrombosis secondary to stent fracture. It is a possible combination of strut exposure, part malapposition and possible thrombogenicity of the drug polymer which culminates in a clinically relevant thrombotic event.
The natural history of stent fracture is associated with ISR and stent thrombosis4,6,12,16. Histological evidence demonstrates neointimal hyperplasia takes approximately four to six weeks to develop17. The majority of stent fractures appear to occur between two and eight months. Few cases have reported fractures occurring after only a few days18. It is believed the early stent fractures are a direct result of mechanical injury related to over expansion with post-dilatation causing injury to cell integrity. Late fractures are more dependent on the local biological processes inducing vessel remodelling or counteractive forces related to the length of stent within a tortuous segment of vessel. Hence, different mechanisms driving stent fracture result in differential timing (acute versus late) of clinical adverse events related to the fracture.
Mechanisms of stent fractures
There are anatomical and mechanical factors that predispose to coronary stent fracture (Figure 6). Anatomically, increased vessel tortuosity and placement within angulated vessel segments has been shown to be a predictor for stent fracture10. Hence the most commonly affected vessels are the RCA>LAD>LCx>SVG and least the LM9. Long stents in angulated vessels tend to straighten the vessel and subsequently are subjected to greater counteractive forces in the vertical axis as the vessel tries to resume its natural angle and shape. Depending on stent conformability and excessive movement of the epicardial artery during systole (the heart beats approximately 100,000 beats over 24 hrs) a focus site develops at the maximum point of bend and is subjected eventually to mechanical fatigue. This results in weakness and increased susceptibility to kink and finally leads to fracture. Mechanically, overlapping and overexpansion of stents lead to increased radial forces weakening the struts, hence causing fracture (Figure 6). Coronary stent design also plays a role in the aetiology of stent fracture. Currently two basic stent designs exist, a closed cell design (sirolimus-eluting stent) and an open cell design (paclitaxel-eluting). The closed stent design (circular interlocking struts), renders the stent more rigid and has less conformability to the movement of a tortuous vessel, and is thus susceptible to strut fracture. The open cell design (adjacent struts attached in a sinusoidal manner forming hinge points), has better conformability but its natural hinge points are subjected to repeated radial forces causing disruption of the stent. Both stent length and design, in addition to vessel anatomy and mechanical forces are associated with the incidence of stent fracture19.
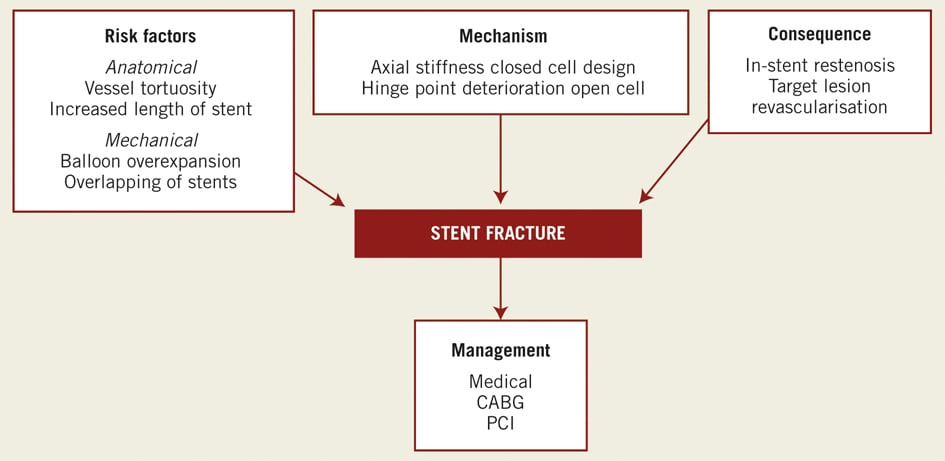
Figure 6. Summary: the clinical sequelae of coronary stent fracture.
The role of intravascular imaging
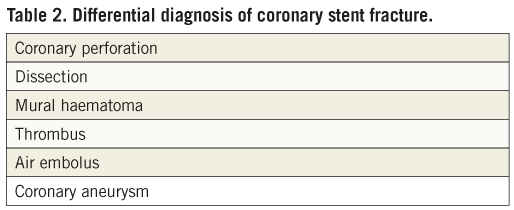
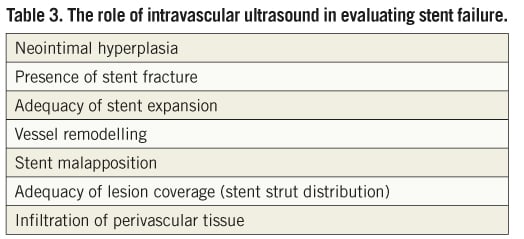
Coronary stent fracture is usually suspected on the basis of an abnormal appearance on angiography post stent deployment/dilatation20. Stent boost technology has also enhanced visualisation of the stent and can aid diagnosis to distinguish fracture from the other differentials (Table 2). Currently the gold standard modality for diagnosis in vivo is IVUS with a resolution of between 150-200 mm compared to that of angiography which has a resolution of 300 mm11. Modern IVUS technology is able to provide detailed characterisation of vessel structure, stent structure and perivascular tissue (Table 3). Although commonly used by the interventionist, the images from IVUS have frequently suffered from artefacts which result in false or erroneous differential diagnosis. Recently intravascular optical coherence tomography (OCT) has detected stent fractures and is now being evaluated to see if its resolution (approximately 10 times that of IVUS) is truly superior compared to other imaging modalities21,22. At present there are anecdotal reports supporting optical coherence tomography (OCT) in the case of suspected stent fracture. Dual source spiral computed tomography and 64 multi-slice computed tomography (MSCT) have been demonstrated to detect stent fractures but have not been evaluated in trials and still pose the added risk of radiation23,24.
Management
There is now evidence showing that stent fractures are associated with a significant higher risk of binary restenosis (37.5%) and TLR (20%)25. However, at present there are no guidelines on the surveillance and management of cases with stent fractures. Currently management decisions are predominantly made at the discretion of the interventionist and are guided by clinical symptoms and signs. Stent fractures not associated with significant intimal hyperplasia have a good prognosis and usually require no further intervention. A number of reports regarding management of stent fracture have been published describing different strategies: plain old balloon angioplasty (POBA), further stenting with BMS, replacing original DES with another, and the use of the same eluting drug but with a different cell design in the context of stent strut fracture26. Often the management decision is conservative. To date, there is no consensus on management of stent fracture. Further analysis of stent cell design associated with stent fracture and long-term follow-up registries of patients with stent fracture are required to define its natural history and guide management.
Conclusion
Coronary stent fractures are increasingly being recognised. Although the data suggests DES and specifically sirolimus-eluting stents due to their more rigid closed cell design, are more commonly implicated in fractures, it is possible that stent fractures in BMS are grossly underestimated. The high prevalence of ISR in the BMS era shifted focus to remedying neointimal hyperplasia while neglecting detailed evaluation of the structural integrity of the stent. Furthermore, neointimal hyperplasia could in theory conceal the strut fractures, making fractures more difficult to appreciate. The earlier generation BMS were also less radio-opaque, and more difficult to visualise on plain angiography compared to modern DES. Due to the development of new stent technology resulting in thinner stent struts and the significant association of stent fractures with late ISR and TLR, further studies with contemporary imaging modalities are needed to study the pathobiology of this increasingly recognised entity.
Conflict of interest statement
The authors have no conflict of interest to declare.
References

