An 82-year-old male patient presented to our department with non-ST-segment myocardial infarction (TIMI score 6). He underwent bypass surgery in 1995. The native proximal circumflex artery had undergone bare metal stenting six months before to treat stable angina (CCS 3).
Emergency angiography identified open venous grafts to the left anterior descending artery, the first diagonal branch, and the right coronary artery (Figure 1). The circumflex artery showed severe proximal in-stent stenosis which was treated by balloon dilatation using up to 18 atm and 3.5 mm diameter balloons. A stent did not pass the lesion and the patient left the table with relevant residual stenosis.
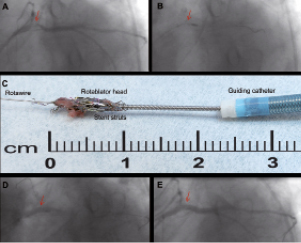
Figure 1. A. Angiographic view of in-stent stenosis in the proximal circumflex artery. B. Trapped rotablator head in the distal portion of the in-stent stenosis. C. Photograph of the rotablator head with the extracted bare-metal stent twisted around it. D. Stent placement, shown during inflation of the stent balloon. E. Final angiographic result. All angiographic images are shown in the “spider view” projection in a caudal left anterior oblique view.
Due to recurrent angina, rotablation of the culprit lesion was performed three days later. After two successful passages of the stenosis with a 1.5 mm rotablator head (160,000 rpm), the rotablator got trapped in the stent. Only submaximal manual force extracted the rotablator head and the guiding catheter. Upon inspection, we found that we had extracted the old stent. Thereafter, we stented the remaining lesion (drug-eluting stent) with a good result. The patient underwent stenting of the LAD bypass due to unstable angina pectoris 10 months after this intervention, with excellent long-term result of the circumflex/left main stent. He is well (CCS 1-2) at 14 months follow-up.
Conflict of interest statement
P. Kirchoff has received honoraria or consultancy fees from 3M Medica/MEDA Pharma, AstraZeneca, Bayer Healthcare, Boehringer Ingelheim, Medtronic, Merck, MSD, Otsuka Pharma, Pfizer / BMS, sanofi-aventis, Servier, Siemens, TAKEDA and received research grants from 3M Medica / MEDA Pharma, Cardiovascular Therapeutics, Medtronic, OMRON, sanofi-aventis, St. Jude Medical, German Federal Ministry for Education and Research (BMBF), Fondation Leducq, German Research Foundation (DFG) and the European Union (EU). H.Reinecke has no conflict of interest to declare.

