CASE SUMMARY
BACKGROUND: A 71-year-old man with a history of coronary bypass surgery in 1998, with left internal mammary artery (LIMA) to left descending artery (LAD) and a saphenous venous graft (SVG) to first obtuse marginal (OM) with jump to posterior descending artery (PDA), was admitted with a non-ST-elevation myocardial infarction and referred for coronary angiography.
INVESTIGATION: Physical examination, laboratory investigations, EKG, transthoracic echocardiogram, coronary angiography.
DIAGNOSIS: Subocclusion of SVG at the junction to PDA at the bifurcation site (Medina 1,1,1) with patency of LIMA to LAD and severe and diffuse native coronary artery disease.
MANAGEMENT: Antiplatelet and antithrombotic therapy, percutaneous coronary intervention (PCI) with dedicated bifurcation stent associated with workhorse drug-eluting stent (DES) via radial approach, scheduled six-month angiographic follow-up.
KEYWORDS: acute coronary syndrome, coronary bifurcation lesion, dedicated bifurcation stent, radial approach, secondary coronary revascularisation
PRESENTATION OF THE CASE
A 71-year-old man was admitted to the emergency room (ER) in May 2011 because of non-ST-elevation myocardial infarction (NSTEMI). He had long-standing arterial hypertension, dyslipidaemia and chronic kidney disease (CKD) with mild renal impairment (GFR 40 ml/min). He had coronary artery bypass graft (CABG) surgery with left internal mammary artery (LIMA) to left anterior descending artery (LAD) and saphenous venous graft (SVG) to first obtuse marginal (OM) with jump to posterior descending artery (PDA) in 1998, because of effort angina.
The patient was free of symptoms and clinical examination was unremarkable. EKG showed inferior ST-segment depression. Serial high-sensitivity troponin levels were increased. An echocardiogram showed normal left ventricular function (0.60) with inferior and posterior wall hypokinesia.
The patient was chronically treated with ASA, beta-blocker, calcium-channel blocker, ACE-inhibitor, nitrate and statin.
A clopidogrel loading dose 600 mg and enoxaparin 6,000 IU were administered in the ER. Low-dose i.v. nitrates and beta-blockers produced a complete regression of EKG abnormalities. Because of baseline CKD, proper hydration was administered and coronary angiography was performed 48 hours later by the left radial approach. Native coronary angiography revealed severe and diffuse native coronary artery disease: left main ostial 75% stenosis, LAD proximal 75% stenosis and mid chronic total occlusion (CTO), left circumflex (LCX) mid CTO after OM origin also with CTO at its ostium (Figure 1A); right coronary artery proximal 75% stenosis and mid CTO (Figure 1B); only partial epicardial Rentrop filling to LCX was present. Angiography of the grafts revealed LIMA patency without any critical LAD stenosis after the junction (Figure 2), and SVG patency to OM with critical stenosis at the junction to the PDA at the bifurcation site (Medina 1,1,1)1 (Figure 3, Moving image 1).
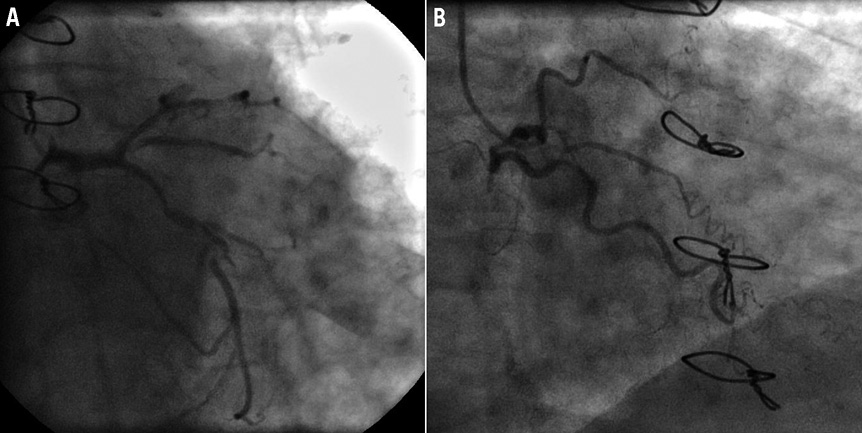
Figure 1. A) Native left coronary artery diffuse disease (CAU 30; RAO 0). B) Native right coronary artery CTO (CAU 0; RAO 30).
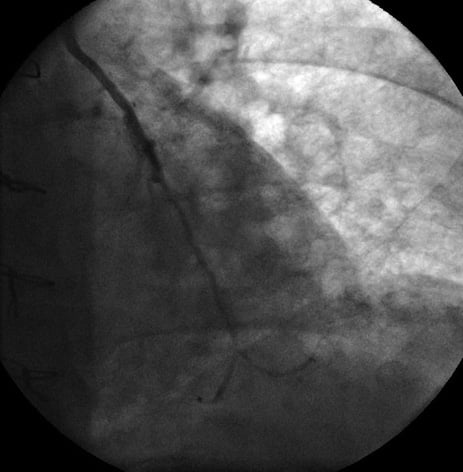
Figure 2. LIMA to LAD patency (CRA 30; LAO 0).
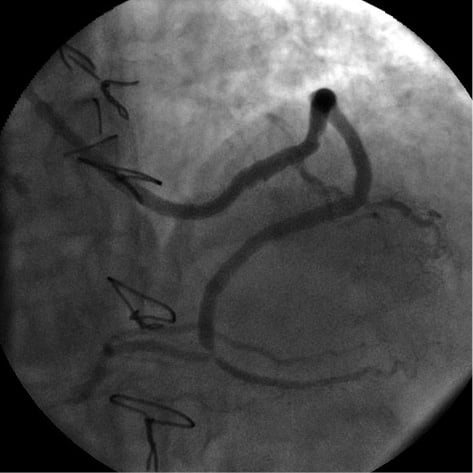
Figure 3. SVG to first OM with jump to PDA (CRA 30; LAO 0) demonstrating subocclusive stenosis at the junction to PDA at the bifurcation site (Medina 1,1,1).
How would I treat?
THE INVITED EXPERT’S OPINION
Long-term event-free survival after coronary artery bypass graft (CABG) surgery will continue to be limited as long as saphenous venous grafts (SVGs) are used as conduits for surgical revascularisation. Patients undergoing CABG are often old and suffer significant comorbidities, as in the case presented.
In this specific patient, CABG had been performed more than 12 years before and he was admitted for non-ST-elevation myocardial infarction (NSTEMI) associated with multiple comorbidities, including renal impairment.
The diagnostic coronary angiography (CAG) done 48 hours after admission revealed well functioning left internal mammary artery (LIMA) to left anterior descending artery (LAD) and patent SVG to OM; however, the anastomotic site to the posterior descending artery (PDA) showed true bifurcational stenosis.
In the setting of inferoposterior wall hypokinesia on echocardiography, ST-segment depression in the inferior leads and the angiographic findings, the SVG-PDA stenosis is presumed to be the culprit lesion. An invasive approach is necessary since the patient already has NSTEMI. Percutaneous treatment seems to be the best choice in this case; however, periprocedural proper hydration in a patient with renal impairment is mandatory during the anticipated complex and time-consuming procedure. Ad hoc PCI would be performed if the contrast volume used in the diagnostic CAG did not exceed the allowed limits; otherwise, PCI can be delayed to avoid contrast-induced nephropathy.
Traditionally, PCI of the native vessel is preferable to that of a graft, especially SVGs, which are often degenerate, since this carries higher acute and long-term risks2.
However, in this particular patient, the culprit lesion requires intervention that involves both the native vessel and the graft. Moreover, recanalisation of the right coronary artery (RCA) chronic total occlusion (CTO) antegradely, or utilising the SVG as a retrograde conduit, is not the first treatment of choice, because of the long segment of CTO to be crossed.
Despite the class I American College of Cardiology/American Heart Association PCI guidelines’ recommendation for the use of embolic protection devices in SVG intervention, the use of distal protection is restricted in the distal lesions because of the absence of a landing zone required to place the device distal to the lesion. A proximal balloon occlusion device would be a good alternative, if available3.
The left radial vascular access utilised in the diagnostic CAG is still a good choice for PCI. Choosing the correct guiding catheter has an important impact on the success rate of SVG intervention. In this patient, I would use a standard sheathless 7 Fr Amplatz left guiding catheter (GC) (Cordis, Johnson & Johnson, Warren, NJ, USA) to get the required back-up and to allow the use of simultaneous double balloons or stents in bifurcational lesions if needed. It has been reported that sheathless transradial PCI using a standard large-bore GC catheter is a safe and effective method for treatment of complex lesions4.
Proper anticoagulation should be achieved throughout the procedure. A floppy coronary guidewire could be advanced through the SVG across the lesion into the PDA distal to the anastomotic site; however, the PDA segment proximal to the anastomosis should also be secured since it supplies a good retrograde filling to the distal right coronary artery (RCA) and the posterolateral branch (PLB). In view of the reversed angle between the SVG and the proximal PDA, wiring can be difficult. A hydrophilic wire would be used after proper shaping of the wire tip. This can also be facilitated by certain tools, such as the Crusade catheter (Kaneka, Osaka, Japan) or the Venture wire control catheter (St. Jude Medical, St. Paul, MN, USA), which is a deflectable tip catheter capable of forming any angle from 0 to 90 degrees in a single plane. Sequential predilatation would be done in both limbs of the bifurcational lesion to prepare for stenting. IVUS study via the two wires would be done after nitroglycerine delivery to assess the size of the PDA and to guide the stenting process. IVUS might reduce the use of frequent contrast injection in such a patient with renal impairment.
A provisional single-stent strategy with a drug-eluting stent would be applied if IVUS showed no severe mismatch between the diameters of the SVG and the distal PDA after predilatation, provided there is no significant residual stenosis in the SVG proximal to the PDA lesion. Moreover, a drug-eluting balloon might be used through the stent struts for this lesion after kissing balloon inflation.
In case of significant mismatch between SVG and PDA diameters, a two-stent strategy with the V-stenting technique would be a reasonable option. With this technique, the wire access to either limb of bifurcation is never lost, so there is no need to cross the stent struts. This reduces procedural time, radiation exposure and contrast use. Selection of stent sizes would also be helped by IVUS guidance.
Conflict of interest statement
The author has no conflicts of interest to declare.
How would I treat?
THE INVITED EXPERT’S OPINION
The patient described in this report is a 71-year-old man with a history of three-vessel coronary artery bypass surgery performed in 1998 who presented with an acute myocardial infarction. The sequential saphenous vein graft (SVG) to diagonal, obtuse marginal and right posterior descending artery/posterolateral artery (PDA) had anastomotic stenosis at the PDA touchdown. The stenosis was discrete and involved both antegrade and retrograde limbs of the PDA branch. The native branch was approximately 2 mm. This stenosis was thought to be the culprit for the myocardial infarction with escalating levels of troponin. There was TIMI 3 flow through the graft and native vessels.
Anastomotic lesions are more often fibrotic lesions and sometimes hard to dilate. The SVG that was thought to be the culprit for myocardial infarction may possibly be associated with a thrombus. While approaching such lesions it is important to keep two things in mind: protection against debris embolisation and compromise to the other limb of the vessel. With non-availability of the proximal protection device Proxis (St. Jude Medical, St. Paul, MN, USA) and inability to deploy satisfactory distal protection because of no landing zone, small vessel size and inability to protect both branches with one device, one has to rely on vasodilators pre and post intervention. Further protection of the other limb can be achieved by keeping the guidewire in the side branch and being ready to access the side branch with a hydrophilic guidewire after the stent placement.
In my opinion, an Amplatz left 1 guide (Cordis, Johnson & Johnson, Warren, NJ, USA) for intubation will ensure good support. I would utilise two guidewires, balance middleweight, and place them in each limb. After pre-treatment with vasodilators like nitroglycerine and nicardipine, a 2 mm balloon catheter dilatation would be very informative regarding the dilatability of the stenosis as well as giving some sense of plaque movement towards the other branch. If the flow is not compromised, I would use a 2.25×15 mm drug-eluting stent from the SVG to the PDA antegrade limb. In case of compromised flow in the retrograde limb or symptoms of angina or ST segment changes or worsening of stenosis to near occlusion, I would cross with a hydrophilic wire to the retrograde limb. This would be followed by balloon dilatation with a 2 mm balloon catheter through the struts. Finally, simultaneous kissing balloon catheter inflation with two 2.0×12 mm balloon catheters would be used to flare up the proximal segment of the stent. This would be followed by post-procedural treatment with intracoronary adenosine.
One should avoid a two-stent technique as an initial approach because of the distal location of the stenosis, small calibre of the native branches, the multiple myocardial segments supplied by the graft and the lack of advantage of the two-stent technique.
Conflict of interest statement
The author has served as a speaker for the Volcano Corporation.
How did I treat?
ACTUAL TREATMENT AND MANAGEMENT OF THE CASE
EKG and echo abnormalities and angiographic findings demonstrated that the culprit lesion was the SVG critical stenosis at the junction to the PDA. After angiography, possible treatment options were considered.
The strategy was to treat such a bifurcation lesion (BL) by use of a Tryton SB stent (Tryton Medical, Durham, NC, USA) in conjunction with a workhorse drug-eluting stent (DES); the bifurcation main branch (MB) was considered to be the part of the PDA in the direction of the RCA, and the distal PDA was considered to be the side branch (SB). A 6 Fr Amplatz left 1 (AL1) GC (Medtronic, Minneapolis, MN, USA) was used for SVG cannulation. Two 0.014 inch Runthrough® NS floppy guidewires (Terumo, Tokyo, Japan) were advanced through the SVG, crossing the bifurcation, into the distal main and side branches; MB and SB predilatation with 3.0×15 mm and 2.5×15 mm Maverick® balloons (Boston Scientific, Natick, MA, USA), respectively, was performed (Figure 4A). A 3.5-2.5×19 mm Tryton SB stent was positioned with the two middle markers equidistant from the bifurcation carina and was deployed at 15 atm (Figure 4B); the side branch guidewire was then retrieved and advanced into the distal MB crossing the Tryton transition zone and allowing the positioning of a 3.0×18 mm Nobori (Terumo) stent which was deployed at 15 atm (Figure 4C). Final kissing was performed by use of a 3.5×15 mm Quantum™ Maverick® balloon (Boston Scientific) in the MB and a 2.5×15 mm Quantum Maverick balloon in the SB; simultaneous inflation was performed up to 20 atm (Figure 4D) with an excellent angiographic result (Figure 5, Moving image 2).
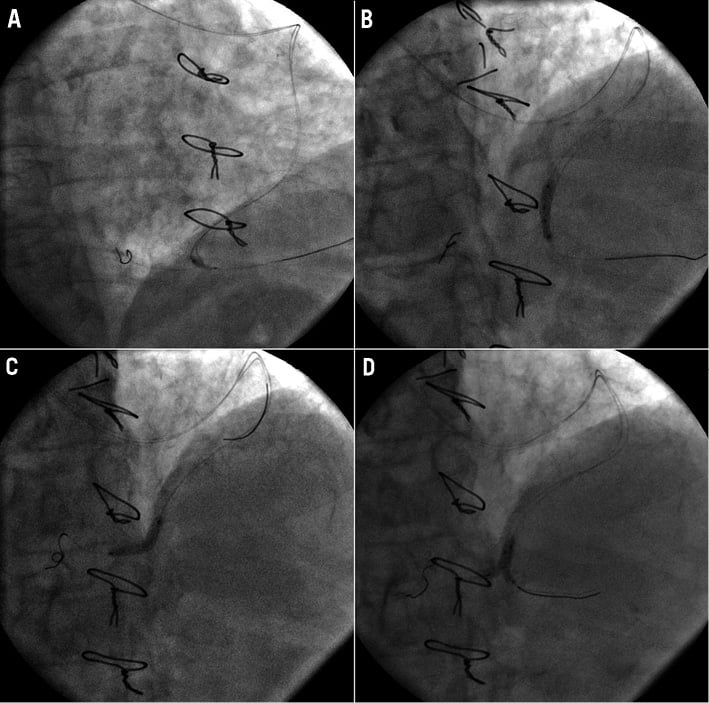
Figure 4. PCI strategy on SVG to PDA. A) MB predilatation with a 3.0×15 mm semi-compliant balloon. B) 3.5-2.5×19 mm Tryton SB stent implantation. C) 3.0×18 mm DES implantation. D) Final kissing with non-compliant balloons.
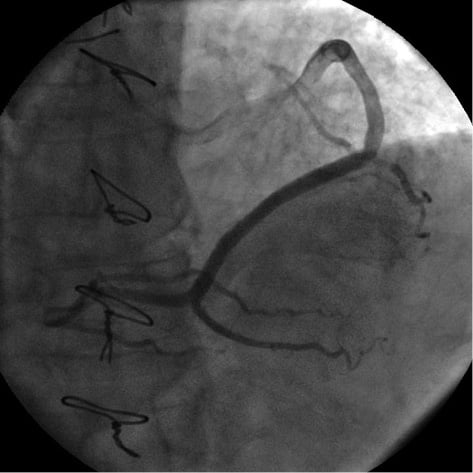
Figure 5. SVG final angiogram (CRA 30; LAO 0).
Intraprocedural treatment with bivalirudin was performed.
No in-hospital adverse events occurred and the patient was discharged with ASA and clopidogrel.
At four-month clinical follow-up, the patient was free of symptoms and dobutamine echo revealed no myocardial ischaemia. At six-month angiographic follow-up no significant ISR was documented (Figure 6, Moving image 3).
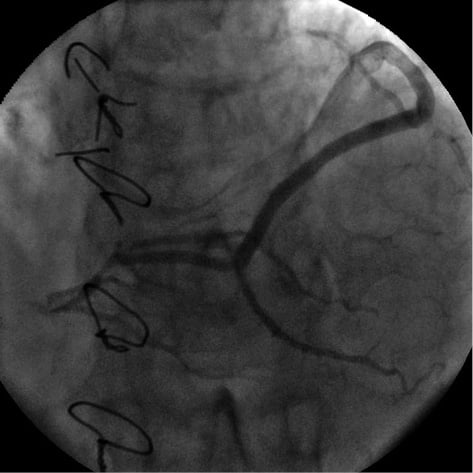
Figure 6. Six-month angiographic follow-up (CRA 30; LAO 0).
Discussion
Major improvements in secondary coronary revascularisation and bifurcation lesion treatment are currently being sought.
Secondary coronary revascularisation, either surgical or percutaneous, as a result of the long-term failure of conduits and stents, or natural native coronary disease progression, has substantially increased in the last decade, carrying a higher risk profile compared to primary revascularisation.
Limited data comparing the efficacy of PCI vs. redo CABG in patients with previous CABG are available5. Because of the higher perioperative and short-term mortality of redo CABG without any benefit in terms of the long-term mortality compared to PCI, ESC guidelines on myocardial revascularisation indicate PCI as the preferred strategy in patients with LIMA patency and amenable anatomy. CABG is preferred in patients with severe graft disease, reduced LV systolic function, more CTOs, as well as absence of a patent arterial graft6.
In the present case, secondary coronary revascularisation was mandatory due to unstable clinical presentation despite optimal medical treatment; LIMA to LAD and SVG to OM branch good patency obviously did not indicate the need for redo CABG surgery.
Therefore, we considered PCI as the only available treatment and different technical approaches were evaluated. CTO recanalisation of RCA appeared difficult because of its persistence for more than ten years, blunt stump with no clear point of entry and no collaterals to allow a retrograde approach. An additional risk due to the need for a higher amount of contrast, particularly in patients with CKD, was considered. Therefore, PCI was performed on the SVG junction to the PDA at the site of a true BL.
Compared with simple lesions, bifurcations are associated with lower procedural success rates, higher adverse event rates and less favourable outcome, mainly due to the inability of current devices and techniques to scaffold the SB ostium, which is a common site of restenosis7.
Randomised trials did not show any clear advantage of routine double stenting over a provisional strategy of MB stenting with only balloon angioplasty of the SB. Double stenting should generally be reserved for true BL and mainly depends on SB characteristics (diameter, territory of distribution, disease location, angle for re-crossing after MB stenting)8,9.
With no strong evidence of superiority of a specific technique in improving patient outcome, selection of the double stenting technique is up to operators. More recently, the introduction of dedicated SB bifurcation stents, specifically designed to secure the SB, may significantly change elective double stenting by facilitating certain techniques and thus making the procedure safer and shorter10.
The Tryton SB stent is a balloon-expandable bare metal stent with a proven high rate of success, and a good safety and efficacy profile when implanted in association with a standard workhorse DES. More than 5,000 patients have been treated with Tryton and more than 1,000 were studied, with over six months of follow-up showing less than 4% TLR and a low thrombosis rate11-14.
A large randomised trial comparing provisional SB stenting with Tryton in true BL is ongoing and will provide further important information concerning BL treatment.
To our knowledge, this is the first report on treatment of a SVG with a Tryton SB stent and could be a useful example showing how to perform a simple and safe procedure on such a distal and complex BL in the context of a secondary coronary revascularisation and with demonstration of good immediate and midterm clinical and angiographic results.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Online data supplement
Moving image 1. SVG baseline angiogram.
Moving image 2. SVG final angiogram.
Moving image 3. Six-month angiographic follow-up.
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 1. SVG baseline angiogram.
Moving image 2. SVG final angiogram.
Moving image 3. Six-month angiographic follow-up.

