A 52-year-old male underwent coronary angiography for non-ST-elevation myocardial infarction. He had a known history of diabetes mellitus and also triple vessel disease with prior coronary artery bypass grafting (CABG) six years ago. Angiography showed total occlusion of the left anterior descending (LAD), circumflex and right coronary arteries (RCA). Left internal mammary graft to LAD was patent. However, there were two critical stenoses in the mid-body of the SVG-RCA and SVG-obtuse marginal (OM) grafts (Figure 1A and Figure 1B). We proceeded to perform percutaneous coronary intervention of both lesions with the prophylactic use of an embolic protection device. After predilation of both lesions, an Absorb™ bioresorbable vascular scaffold 3.0×18 mm (Abbott Vascular, Santa Clara, CA, USA) was implanted in the mid-body of the saphenous vein graft (SVG)-RCA (Figure 1C) whereas a XIENCE PRIME™ 3.0×23 mm stent (Abbott Vascular) was implanted in the mid-body of the SVG-OM (Figure 1D). The final angiographic results were good and there was no in-lab complication. He presented three months later with atypical chest pain and underwent cardiac computed tomographic angiography (CCTA). Both bioresorbable vascular scaffold (BVS) and XIENCE PRIME stent were widely patent on CCTA (Figure 1E and Figure 1F). No blooming artefact was observed within the treated SVG-RCA segment with the BVS platinum markers clearly seen on CCTA.
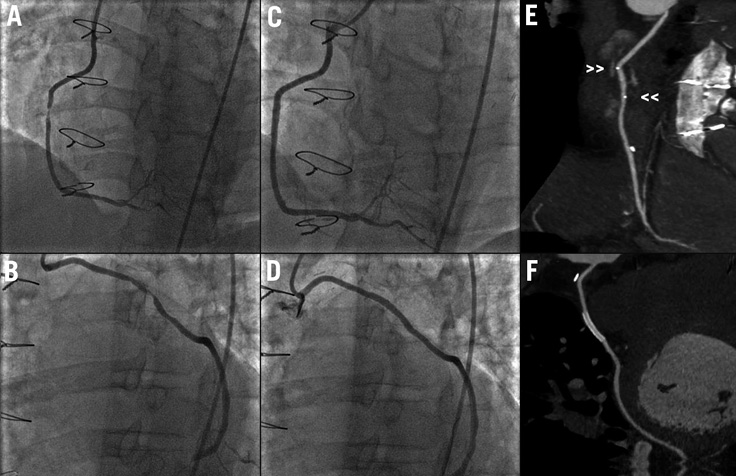
Figure 1. A) Baseline angiography showing critical stenosis in mid-body of SVG-RCA. B) Baseline angiography showing critical stenosis in mid-body of SVG-OM. C) Final angiography of SVG-RCA (after successful deployment of Absorb). D) Final angiography of SVG-OM (after successful deployment of XIENCE stent). E) CCTA showing widely patent BVS in mid-body of SVG-RCA. No blooming artefact observed with the BVS platinum markers clearly seen (small arrowheads >>) on CCTA. F) CCTA showing widely patent XIENCE metallic stent in mid-body of SVG-OM.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Online data supplement
Moving image 1. Movie clip showing the appearance of BVS in the mid-body of the SVG-RCA on CCTA.
Moving image 2. Movie clip showing the appearance of XIENCE stent in the mid-body of the SVG-OM on CCTA.
Supplementary data
To read the full content of this article, please download the PDF.

