An 81-year-old woman presented with non-ST segment elevation myocardial infarction (NSTEMI). One-vessel disease was diagnosed: type C, 80% stenosis in the mid left anterior descending artery (LAD), distal to a bare metal stent (BMS) implanted in 2002 in the proximal-mid LAD, and further treated with brachytherapy in 2003 due to in-stent restenosis (Figure 1A, Moving image 1). The patient underwent implantation of an Absorb Bioresorbable Vascular Scaffold (BVS) (3.0×28 mm) (Abbott Vascular, Santa Clara, CA, USA), which overlapped with the BMS, in the mid-distal LAD, followed by post-dilatation (3.25×20 mm non-compliant balloon at 20 atm). The final result was optimal (Figure 1B, Moving image 2). Fourteen months after BVS implantation, six days after stopping clopidogrel, the patient presented with anterior STEMI. Abundant thrombus was retrieved (Figure 1C, Moving image 3). Optical coherence tomography (OCT) was then performed and ruled out edge dissection, BMS and BVS fracture and underexpansion/malapposition (Figure 1D, Moving image 4). Residual strut-adherent thrombotic material was found at the BVS-BMS overlap, where incomplete tissue coverage was observed (Figure 1E). A metallic everolimus-eluting stent was implanted and post-dilated, with a good result (Figure 1F, Moving image 5, Moving image 6). Mechanisms underlying very late scaffold thrombosis are discussed in the Online Appendix.
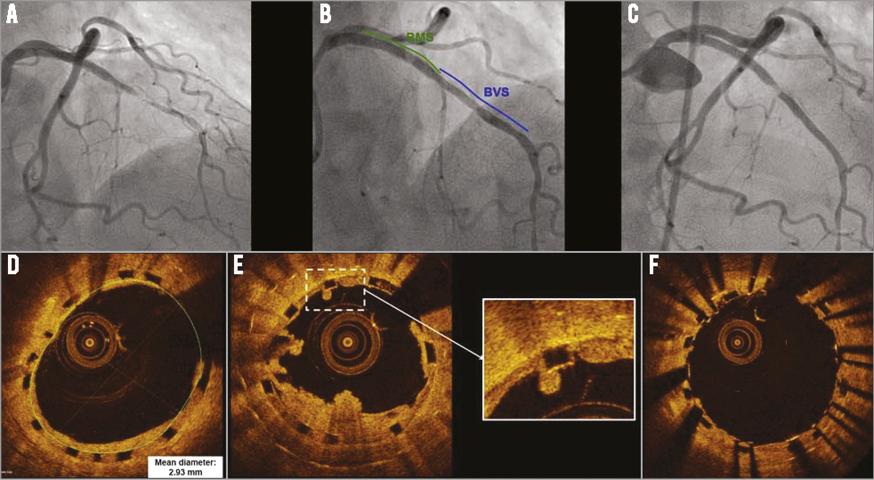
Figure 1. Bioresorbable vascular scaffold implantation and very late thrombosis. A) Pre-treatment angiogram. Final result was optimal (B). Fourteen months later, the patient presented with anterior STEMI. After thrombectomy (C), OCT was performed (D). Thrombotic material was found at the BVS-BMS junction (E), where incomplete tissue coverage was also observed (zoom). (F) Final result.
Funding
L. Azzalini is funded by a grant of the Spanish Society of Cardiology. P. L’Allier is supported by La Fondation de l’Institut de Cardiologie de Montréal and holds the Des Groseillers-Bérard Chair in Interventional Cardiology.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Online Appendix. Mechanisms underlying very late scaffold thrombosis pathophysiology
In the early phase after implantation, the pathophysiology underlying stent thrombosis is mainly related to mechanical problems and issues with dual antiplatelet therapy (DAPT)1. However, predictors of very late stent thrombosis (VLST) are not well documented. Late incomplete stent apposition due to excessive positive remodelling (due to persistent inflammation), incomplete stent endothelialisation and development of in-stent neoatherosclerosis with plaque rupture have been suggested as potential mechanisms1. No specific data on VLST with BVS exist. Persistent inflammation is not expected to play a major role, since BVSs begin to reabsorb soon after implantation, a process that does not involve inflammation and is completed in 18-24 months2. Incomplete tissue coverage was identified as the main contributing factor of VLST in our patient, although it must be noted that the complex scenario in which the BVS was implanted might have played a role (the BVS was deployed, partially within a BMS, to treat distal atherosclerotic disease progression after brachytherapy). Recently, Karanasos et al3 reported the first case of VLST, two years after Absorb implantation. This was also associated with discontinuation of DAPT (four days earlier), although the major mechanism contributing to VLST appeared to be scaffold disruption with post-dilatation. Both cases suggest that longer DAPT duration (i.e., >24 months) should be considered, when BVSs are implanted to treat complex lesions. Development of in-scaffold neoatherosclerosis with subsequent plaque rupture is a third potential mechanism underlying VLST. We observed a case of VLST (22 months after implantation) in the setting of moderate diffuse in-scaffold restenosis (39% by quantitative coronary angiography; documented four months before VLST). Unfortunately, intravascular imaging was not performed at the time of VLST, and the underlying mechanism could not be confirmed. Due to their reabsorption, BVSs were believed to be almost immune from VLST. However, these recent reports suggest otherwise. Therefore, it seems prudent to obtain long-term safety data prior to widespread clinical utilisation of BVS.
Online data supplement
Moving image 1. Pre-treatment angiogram.
Moving image 2. Result after 3.0×28 mm Absorb BVS implantation.
Moving image 3. Very late BVS thrombosis. Result after initial thrombectomy.
Moving image 4. OCT after initial thrombectomy.
Moving image 5. OCT after DES implantation and post-dilatation.
Moving image 6. Final angiographic result.
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 1. Pre-treatment angiogram.
Moving image 2. Result after 3.0×28 mm Absorb BVS implantation.
Moving image 3. Very late BVS thrombosis. Result after initial thrombectomy.
Moving image 4. OCT after initial thrombectomy.
Moving image 5. OCT after DES implantation and post-dilatation.
Moving image 6. Final angiographic result.

