
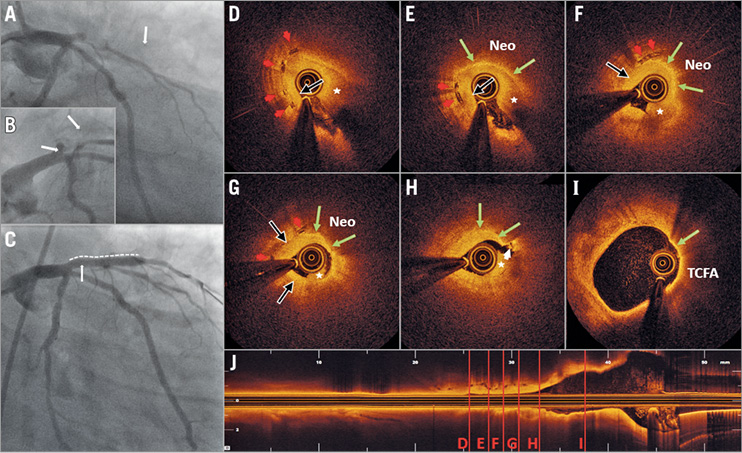
A 31-year-old male who had crescendo angina for three weeks experienced a non-ST-elevation myocardial infarction (CK-MB 8.7, troponin I 0.24 ng/mL) due to an in-scaffold proximal left anterior descending occlusion 30 months after uncomplicated implantation of a 3.5×18 mm Absorb™ bioresorbable vascular scaffold (BVS; Abbott Vascular, Santa Clara, CA, USA) in a soft tissue culprit lesion. Platinum BVS markers are indicated (white arrows) (Panel A, Panel B, Moving image 1). After gentle predilation, optical coherence tomography (ILUMIEN™ OPTIS™; St. Jude Medical, St. Paul, MN, USA) revealed extensive lipid deposition with thin-cap fibroatheroma (TCFA) (Panel I, Moving image 2). Cross-sectional levels are indicated (Panel J). Residual thrombus (asterisk) and suboptimal blood clearance due to restenosis precluded optimal image acquisition. Critical BVS restenosis consisted of homogeneous neointimal hyperplasia (black arrows) adjacent to areas with heterogeneous neointima (Neo) and accumulations of macrophages (green arrows), suggesting neoatherosclerosis as the underlying mechanism of scaffold failure (Panel D-Panel H). Remnant scaffold struts (red arrowheads), visible 30 months after implantation, did not suggest late structural discontinuity. White thrombus (white arrowhead) adjacent to a TCFA was noticed, although predilation precludes a definite diagnosis of plaque rupture (Panel H). Implantation of a 3.0×26 mm DES (dotted line) and post-dilation using a 3.5 mm non-compliant balloon showed an optimal result (Panel C, Moving image 3).
Larger OCT series did not identify neoatherosclerosis as an underlying mechanism in late/very late BVS failure. However, delayed scaffold bioresorption in diseased coronary arteries might cause higher than expected low-grade hypersensitivity reactions and peri-scaffold inflammation, resulting in neoatherosclerosis in BVS. Neoatherosclerosis might become a conceptually unexpected mechanism of late/very late scaffold failure. Long-term follow-up is indicated.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary data
Moving image 1. Angiography at presentation showing a thrombotic occlusion of the proximal left anterior descending coronary artery, within the previously implanted 3.5×18 mm bioresorbable vascular scaffold (BVS) (Absorb; Abbott Vascular). Right anterior oblique view with caudal inclination.
Moving image 2. Optical coherence tomography (ILUMIEN OPTIS; St. Jude Medical) after gentle predilation with a 2.0 mm balloon, revealing extensive lipid accumulation with TCFA in the proximal LAD coronary artery, without clear evidence of plaque rupture. Critical BVS restenosis was seen, consisting of areas with homogeneous neointimal hyperplasia, adjacent to areas with more heterogeneous neointima with high signal attenuation and accumulation of macrophages suggesting the presence of neoatherosclerosis. Mural white thrombus in an area with TCFA can be seen within the BVS, although predilation and the presence of residual thrombus precludes the definite diagnosis of a neoatherosclerotic ruptured TCFA. Remnant scaffold struts, still visible 30 months after implantation, did not suggest late structural discontinuity.
Moving image 3. Angiography after predilation with a 2.0 mm balloon and implantation of a 3.0×26 mm drug-eluting metallic stent. Post-dilation was performed using a 3.5 mm non-compliant balloon, restoring TIMI 3 flow. Right anterior oblique view with caudal inclination.
Supplementary data
To read the full content of this article, please download the PDF.
Angiography at presentation showing a thrombotic occlusion of the proximal left anterior descending coronary artery, within the previously implanted 3.5×18 mm bioresorbable vascular scaffold (BVS) (Absorb; Abbott Vascular). Right anterior oblique view with caudal inclination.
Optical coherence tomography (ILUMIEN OPTIS; St. Jude Medical) after gentle predilation with a 2.0 mm balloon, revealing extensive lipid accumulation with TCFA in the proximal LAD coronary artery, without clear evidence of plaque rupture. Critical BVS restenosis was seen, consisting of areas with homogeneous neointimal hyperplasia, adjacent to areas with more heterogeneous neointima with high signal attenuation and accumulation of macrophages suggesting the presence of neoatherosclerosis. Mural white thrombus in an area with TCFA can be seen within the BVS, although predilation and the presence of residual thrombus precludes the definite diagnosis of a neoatherosclerotic ruptured TCFA. Remnant scaffold struts, still visible 30 months after implantation, did not suggest late structural discontinuity.
Angiography after predilation with a 2.0 mm balloon and implantation of a 3.0×26 mm drug-eluting metallic stent. Post-dilation was performed using a 3.5 mm non-compliant balloon, restoring TIMI 3 flow. Right anterior oblique view with caudal inclination.

