Abstract
Bioresorbable scaffolds (BRS) are a promising new interventional treatment strategy for coronary artery disease (CAD). They are intended to overcome some of the shortcomings of metal drug-eluting stents (DES), mainly late reinterventions which occur at a consistent rate after one year and have not been reduced by the use of local drug elution. Initial experience in non-complex lesions established efficacy in opening the vessel and the concept of bioresorption. However, with the use of BRS in more complex lesions, the incidence of BRS failure, including both scaffold restenosis and thrombosis, has also increased. Therefore, understanding of both the pathophysiology and of the available treatment options of scaffold failure remains an important issue in ensuring procedural and long-term clinical success.
Introduction
Since the introduction of drug-eluting stents (DES), the rates of in-stent restenosis (ISR) and target lesion revascularisation (TLR) during the first year have decreased significantly compared to those of bare metal stents (BMS). However, after one year, both stent thrombosis (ST) and restenosis still occur, most probably caused by in-stent neoatherosclerosis due to biocompatibility issues of foreign materials (polymers and metallic components of DES). To improve the long-term outcome, fully bioresorbable scaffolds (BRS) have been developed which leave no foreign materials and allow the restoration of normal coronary physiology, positive remodelling of the atherosclerotic vessel, non-invasive imaging and full pharmacological percutaneous and surgical treatment options if symptoms should reoccur.
In recent years, bioresorbable scaffolds (BRS) have evolved as the new treatment strategy for coronary artery disease (CAD) with the Absorb Vascular Scaffold (BVS; Abbott Vascular, Santa Clara, CA, USA) being the device most intensively studied. The Absorb BVS system consists of a poly-L-lactide (PLLA) bioresorbable backbone with a poly-DL-lactide (PDLLA) coating that releases the antiproliferative drug everolimus. PLLA and PDLLA are degraded via hydrolysis of the ester bonds, and the resulting lactate and its oligomers are metabolised by the pyruvate and Krebs energy cycles. The strut thickness is 156 µm1.
A second CE-marked scaffold, the DESolve™ novolimus-eluting bioresorbable coronary scaffold system (Elixir Medical Corporation, Sunnyvale, CA, USA) is currently under investigation and very little is known about its performance in real-world patients2. Although the DESolve is also PLLA-based, the degradation, and drug-elution profile is different, and different timings for failure strategies may apply.
Using invasive imaging at two years, it was demonstrated that Absorb BVS are largely absorbed and late lumen enlargement occurred. In this way, BRS offer transient vessel support to prevent acute vessel recoil during angioplasty while eluting an antiproliferative drug to minimise neointimal hyperplasia during the healing process. Several clinically oriented studies (ABSORB EXTEND, ABSORB II) in non-complex patients have shown good results3,4.
However, in other real-world lesion registries5,6 BRS failure still occurred including both ST and scaffold restenosis (Figure 1). In this review we will try to give a short overview of the pathophysiology and the treatment options in case of BRS failure.
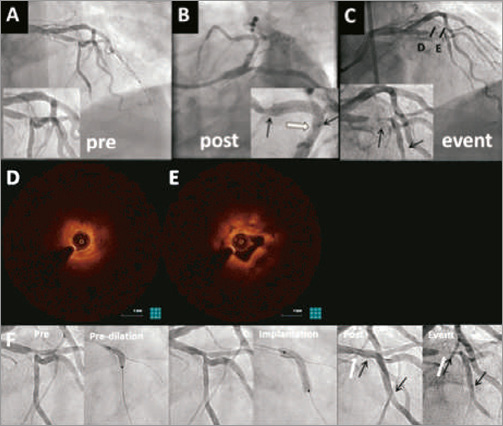
Figure 1. Edge restenosis treated with Absorb BVS. A 65-year-old male patient presenting with an NSTEMI was treated with a 3.5×28 mm Absorb BVS for a trifurcation lesion of the LAD and two diagonals (A & B). He returned 142 days later for unstable angina due to a subtotal occlusion of the LAD with slow flow distal to the scaffold (TIMI 1) (C). OCT imaging revealed edge restenosis as the underlying mechanism for BRS failure (D) and restenosis within the scaffold with a layered pattern (E), but no luminal thrombus. The patient was treated with thrombus aspiration and a 3.5×38 mm DES (PROMUS™; Boston Scientific, Marlborough, MA, USA). A retrospective review of the post-procedural angiogram at baseline showed proximal edge dissection (B) and incomplete lesion coverage with geographic miss as the reason for restenosis (F series). Black arrows indicate the scaffold markers and white arrows the uncovered edge segment. Adapted from Antonis Karanasos et al; Angiographic and optical coherence tomography insights into bioresorbable scaffold thrombosis. A single-center experience. (Accepted and in press Circ Cardiovasc Interv 2015).
Risk factors for scaffold restenosis
The mechanism for BMS or DES restenosis is multifactorial and consists of stent recoil, formation of neointima, organisation of thrombus, geographical miss and vessel remodelling. The pivotal factor in the process of ISR is neointimal formation, due to migration and proliferation of smooth muscle cells and myofibroblasts. In the long term, metallic DES might fracture at hinging points in the coronary artery inducing an inflammatory reaction. Occasionally, some patients seem to be “limus” resistant and develop early restenosis7 (Figure 2). Finally, negative remodelling of the vessel contributes to the restenosis process8.

Figure 2. Absorb BVS and neointimal hyperplasia treated with DES, and recurrent failure. A 59-year-old male patient was treated with one Absorb BVS (3.5×28 mm) in the proximal LAD for unstable angina (A-C). The patient developed an NSTEMI 112 days after the index PCI with TIMI 1 flow (D) and was therefore classified as a definite ST. OCT showed mild scaffold underexpansion (3 mm diameter) with severe neointima development (D’ and D’’’) but also areas with late malapposition and potential vasodilatation and thrombus resorption (D’’). Treatment consisted of thrombectomy, eptifibatide and a 3.5×32 mm DES (PROMUS; Boston Scientific) followed by post-dilatation with 4.0 mm balloon, the lumen increased significantly and malapposition was resolved on OCT (E’) with good angiographic results (E) . The patient returned almost four months later with unstable angina. There was a severe ISR on angiography (F) with total occlusion (arrow) and collateral flows suggesting a resistance to the “limus” drugs used. It was decided to perform a semi-urgent CABG, which took place four days later.
The rates of BMS ISR have been described as being as high as 60%, depending on several risk factors such as lesion complexity, patient comorbidities and vessel size9-12. The use of DES has significantly reduced the rate of ISR, although DES ISR rates at one year have been stated as occurring in 3%-20% of patients, depending on DES generation and patient, lesion and procedural characteristics13.
Multiple patient, lesion and procedure-related risk factors for ISR in BMS and DES have been reported, including diabetes mellitus, multivessel disease, stent length, bifurcation lesions, small calibre vessels, chronic total occlusion (CTO), strut thickness, usage of multiple stents and stent underexpansion. Hypersensitivity reaction to the polymer is another important mechanism. ISR by itself is also a predictor for future ISR13-18. In addition, the stent type plays an important role which can be related to strut thickness, drug dosage and drug release profile. In general, thicker stent struts cause more flow disturbances with reduced endothelial shear stress19, which enhances the process of neointimal hyperplasia.
It seems likely that most risk factors for ISR with BRS are the same as for ISR with BMS or DES; however, at this point in time, there is little evidence to confirm this presumption. Recently, a case series reported geographical miss and scaffold underexpansion as being the most frequent causes of BRS failure20.
Risk factors for scaffold thrombosis
A number of risk factors for ST have been described. Many of them are also predictive of stent restenosis. These risk factors can be categorised as lesion, patient and procedure-related factors. Procedure-related factors are stent malapposition, stent undersizing, dissection, placement of multiple stents, stent overlap and stent length. Lesion-related factors include coronary bifurcations, heavily calcified lesions, long lesion length, small vessel size and CTO. Finally, there are patient-related factors such as diabetes mellitus, advanced age, renal failure, low ejection fraction, smoking, prior CABG, acute coronary syndromes (ACS) at presentation, (early) discontinuation of DAPT or resistance to clopidogrel21,22.
Probably, and in line with DES, the rate of BRS ST varies depending on lesion, patient and procedure-related characteristics. The most remarkable difference between BRS and current DES is the increased strut thickness and width (compared to old stainless steel BMS and first-generation DES). This will increase the early uncovered surface significantly. Also, strut thickness induces convective flow patterns, triggering platelet deposition23. Susceptibility to platelet aggregation might be further aggravated in conditions such as scaffold underexpansion, treatment of thrombotic lesions, e.g., during ACS, and DAPT interruption.
Scaffold underexpansion is an issue with BRS and is an important contributor to BRS failure. It is less if lesions are adequately predilatated with balloons on a 1:1 ratio to the vessel size24. Discontinuation of DAPT and edge dissections have also been described as causes of BRS ST25. Currently ongoing and future all-comer, randomised controlled trials will indicate whether BRS have more favourable rates of late ST compared to current-generation DES.
Scaffold dislodgement
In severely calcified or tortuous lesions, successful delivery of BRS can be difficult: the scaffold could be potentially dislodged26 in the same way as metallic stents. However, apart from an early publication, no further cases of scaffold dislodgement have been reported.
Incidence of scaffold failure in BVS studies and registries
Very little is known about the exact incidence of ST and ISR with the use of BRS. In most publications the cause of BRS failure, whether by ST or ISR, is not clearly reported.
Recently, an interim analysis of the ABSORB II study reported a TLR rate at one year of 1% in the Absorb BVS group compared to 2% in the DES group3. The five-year TLR rate in low-risk patients and non-complex lesions of the ABSORB cohort A study was 3.4%27, whereas the TLR rate of an everolimus-eluting stent (EES) (XIENCE; Abbott Vascular) at five years was 8.6% in the SPIRIT III trial28.
Wohrle et al reported on the one-year outcomes of the ASSURE registry: five cases of TLR (2.8%) occurred, all due to ISR. Treatment options used were DEB (two patients with long lesions in small vessels, treated with overlapping BRS), DES (ISR of a saphenous vein graft due to malapposition of the BRS), POBA (for incomplete [proximal] BRS expansion) and CABG (total occlusion of the target vessel)6.
The GHOST-EU trial, including 1,189 patients, showed a TLR rate of 2.5% at six months and target lesion failure (TLF: a composite of cardiac death, target vessel myocardial infarction or ischaemia-driven target lesion revascularisation) rate of 4.4% at six months. In a multivariate analysis, TLF was seen more in patients with diabetes and in smokers; however, this was not statistically significant5.
Ishibashi et al summarised the rates of Absorb BVS ST reported in multiple trials. The incidence of ST varied from 0% up to 3.0% in a time period ranging from one to six months29. In another recent review article the number of definite ST ranged from 0% at one year to 3.2% at six months30.
Most cases of BRS ST occur within the first 30 days after implantation; however, some cases of late ST have also been described. The cumulative incidence of ST at six months was 2.1% in the GHOST-EU trial. In 13% of the cases there was DAPT discontinuation. Other possible causes are the low rate of post-dilatation and little usage of invasive imaging in B2/C lesions5.
Regarding the first 450 patients enrolled in the ABSORB EXTEND trial, seven cases of Absorb BVS failure occurred, i.e., three cases (0.67%) of scaffold dislodgement and four cases of ST (0.89%)26.
Of the 101 patients included in the BVS cohort B trial, only six cases of ISR occurred (5%) during a three-year follow-up period. The mechanisms for ISR were procedural edge injury during the initial procedure, geographical miss, and in one case myocardial bridging. In three cases the cause of ISR could not be identified31.
In brief, TLR rates can be as high as 3% at one year, depending on lesion and patient complexity. BRS failure can be caused by geographical miss, scaffold dislodgement, scaffold malapposition and underexpansion, lesion length and DAPT discontinuation.
How to treat BRS failure
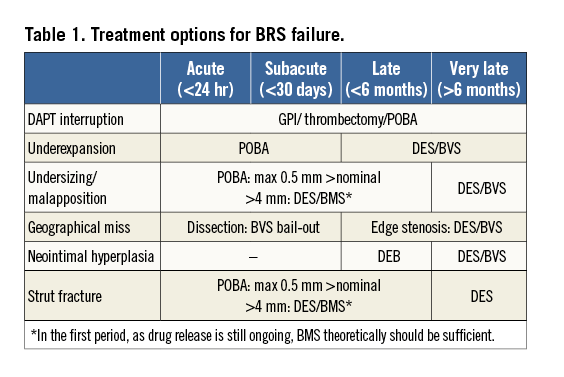
Multiple treatment options for treating BRS failure exist: thrombus aspiration, plain old balloon angioplasty (POBA), BMS, DES, BRS, drug-eluting balloons (DEB) or medical treatment (e.g., with a thrombolytic agent or a glycoprotein IIb/IIIa inhibitor [GPI]). Deciding which is most suitable depends on the triggering mechanism, and not infrequently multiple underlying factors are present. Understanding the fundamental pathophysiological mechanism underlying the TLF is of key importance to direct subsequent management. Invasive imaging modalities, such as optical coherence tomography (OCT) are of paramount importance in order to achieve treatment success. OCT enables the operator to determine between the different mechanisms for BRS TLF such as scaffold underexpansion, scaffold malapposition or undersizing, geographic miss (edge dissection, edge restenosis), neointimal hyperplasia or scaffold strut fracture.
BRS thrombosis after DAPT interruption, whether acute (<24 hours), subacute (<1 month) or late, can be managed with the use of thrombectomy, GPI and/or POBA. In patients on clopidogrel who present with an occlusion of the target vessel due to a thrombus, platelet function testing and switching to a more potent P2Y12 inhibitor has to be considered.
Early underexpansion and malapposition can be treated with POBA with non-compliant balloons of sufficient diameter and pressure, although the maximum overexpansion limit of 0.5 mm always has to be respected for Absorb BVS, especially in the situation of undersizing (Figure 3). If the vessel is above 4 mm in diameter and there is serious malapposition, large metallic stents are indicated. Preferably a DES is used, although potentially a BMS could be sufficient for treatment of acute or subacute (<30 days) scaffold failure. However, the negative effects of an additional dose of antiproliferative drugs when DES are used seem only theoretical and hence not of clinical importance. If underexpansion cannot be managed by POBA alone, a BMS or DES is indicated to ensure additional radial support (Figure 4). To minimise stent overlap, only the insufficiently apposed areas needed be covered with the new stent. After thirty days we strongly recommend DES (or even second BRS in larger vessels) as the remaining dose of everolimus on the BRS might not be sufficient to effectively reduce neointimal hyperplasia (Figure 4). For undersizing, POBA could be sufficient up to six months as the goal is to ensure optimal apposition without further vessel dilatation (low pressure) inducing a new healing process.
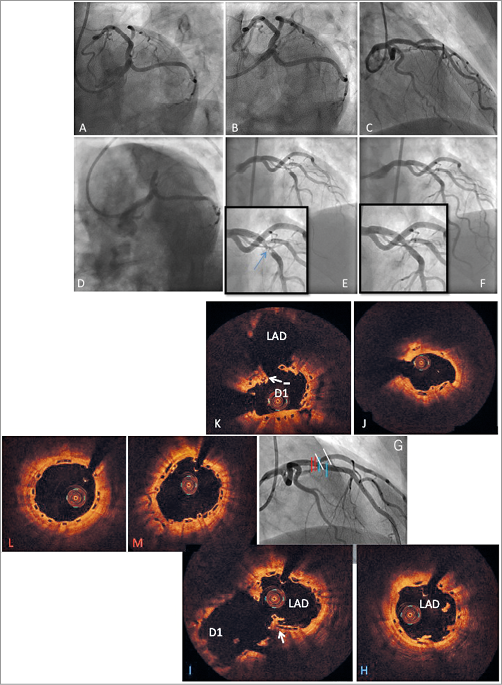
Figure 3. Absorb BVS failure due to discontinuation of DAPT treated with thrombus aspiration and POBA. A 60-year-old male patient with a history of smoking, hypertension and heart failure presented with stable angina. There was one-vessel disease and a LAD, 1st diagonal, lesion (Medina 0,0,1) on angiography. A) Two 3.0×12 mm Absorb BVS were placed in the LAD and 1st diagonal, using the T and protrusion technique with good results in the spider (B) and RAO (C) projections. After 129 days the patient developed a STEMI due to an occluded LAD (D) potentially due to ascal and prasugrel discontinuation for CVA. POBA with a 3.0 mm balloon was then performed. After three AngioJet (Boston Scientific) runs, the angiographic result was acceptable (E) and eptifibatide was continued for 24 hours. The treatment of BRS failure was reviewed four days later using OCT (F-M). Pullback from the LAD (lower row) showed some remaining thrombus (H), and signs of fractures or double layer of uncovered struts (I, arrow). Pullback from the diagonal branch showed some undersizing distal (J) and some double layer and lost struts (K, arrow). Proximal to the bifurcation the struts were well apposed and mainly well covered (L and M).
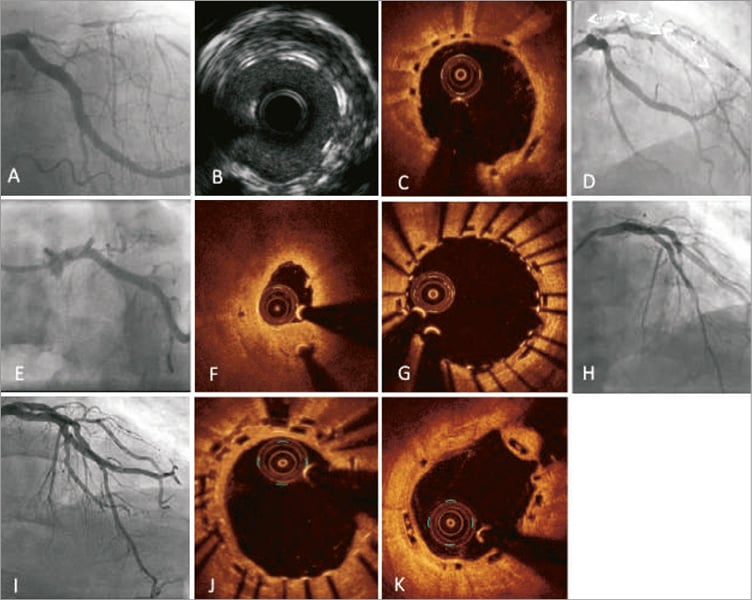
Figure 4. Scaffold thrombosis due to underexpansion treated with DES. A 69-year-old male patient presented with an NSTEMI. Angiography showed one-vessel disease with long narrowing of the proximal and mid LAD and collateral filling (A). Three Absorb BVS (3.0×28 mm, 3.5×18 mm, 3.5×18 mm) were implanted (D). After placement of the first two scaffolds there was compression and thrombus in the 1st diagonal. Invasive imaging post procedure revealed organised thrombus behind the struts of the proximal scaffold (B: IVUS) and thrombus protrusion at the overlapping scaffolds (C: OCT). After 47 days the patient presented with a non-Q-wave myocardial infarction due to a full occlusion in the proximal LAD (E). There was some underexpansion, but a large thrombus on OCT (F). He was treated with thrombectomy, eptifibatide and PCI with a 3.5×38 mm DES (XIENCE) covering the proximal BVSs with a good angiographic and OCT result (G & H). The control diagnostic angiography made 110 days later (I) displayed good scaffold and stent apposition on OCT with good coverage of the struts of the new DES (J) and the untreated original distal Absorb BVS (K).
After approximately six months the tie chains between the crystal polylactide lamellae become more and more hydrolysed and the radial strength and subsequent vessel support gradually decreases32 (Figure 5). BRS failure after six months due to mechanical problems will need placement of an additional stent or scaffold.
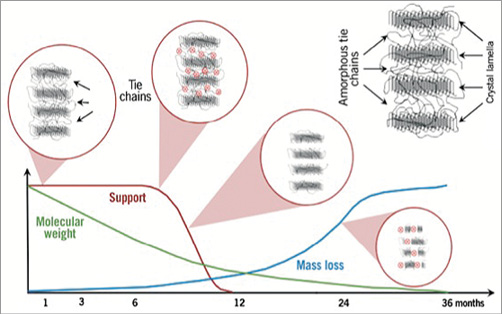
Figure 5. Bioresorption of Absorb BVS. Initially, cleavage of polylactides results in minimal molecular weight loss with remaining full support until six months. After six months, degradation significantly impacted on tie chains between crystal lamellae occurs rapidly, reducing radial support when the material starts to become brittle. Implantation of additional vessel supportive therapy is indicated to successfully treat lumen reduction. Adapted from Serruys et al34.
In the setting of a geographical miss leading to a clinically relevant acute or subacute edge dissection, a BRS bail-out strategy could be used. In case of a geographical miss with apparent edge restenosis, placement of an additional BRS is possible, although converting to DES with a minimal risk of repeat ISR is more prudent. ISR due to intimal hyperplasia can be treated by a DEB (<6 months), but after six months additional vessel support is indicated (with a preference for DES) (Figure 1).
Lastly, in the case of limited scaffold strut fracture, POBA should be able to correct the malapposed segments33. For more extensive fractures or large diameter vessels, a new stent (BMS or DES) would be the treatment of choice. Again, after six months disintegration of the scaffold is initiated and additional radial strength is necessary (DES preferred). In case of both fracture and underexpansion, lesion dilatation is necessary: we recommend an additional DES from thirty days after the initial BRS placement. Treatment options for BRS failure are summarised in Table 1.
However, it must be mentioned that most of the clinical experience with BRS failure has been gained from experience with the Absorb BVS platform, and that, given the paucity of trial numbers, only limited data are available for other BRS subtypes such as the DESolve novolimus-eluting bioresorbable coronary scaffold system (Elixir) or metal-based (magnesium) resorbable devices. As such, these recommendations for the treatment of BRS failure are only applicable to the Absorb BVS.
Conclusion
Treatment of scaffold failure should target any suboptimal result. After thrombus aspiration and aggressive medical treatment, intravascular imaging is advised to reveal any scaffold abnormalities. A wide range of strategies can be applied to correct suboptimal scaffold results. The major difference between BRS and DES in the treatment of target lesion failure is the more frequent need for additional vessel support (using a second BRS or a DES).
Acknowledgements
The authors wish to thank Antonis Karanasos for kindly providing the images for Figure 1.
Conflict of interest statement
R-J. van Geuns and N. Jepson have received speaker’s fees from Abbott. The other authors have no conflicts of interest to declare.