Abstract
Bioresorbable scaffolds promise to counteract late thrombosis by the absence of residual foreign material over time and the restoration of functional endothelial coverage. However, although currently available data are controversial, initial post-marketing studies have raised some concerns about the putative increased early thrombogenicity of bioresorbable scaffolds as compared to currently available second-generation drug-eluting stents. This article focuses on incidence rates, putative mechanisms and prevention strategies of scaffold thrombosis.
Introduction
Stent thrombosis is the most feared complication of percutaneous coronary intervention (PCI) with metallic stents due to its documented association with short-term mortality in ~20-40% and myocardial infarction in ~50-70% of cases. Such variability is dependent on several circumstances, including prompt recognition and management, extent of myocardium exposed to ischaemia, and recruitability of collateral circulation1,2. Drug-eluting stents (DES) are known to delay the endoluminal healing at the angioplasty site, resulting in a rare but persisting stimulus to stent thrombosis that has been diminished but not abolished by second-generation DES3.
Bioresorbable scaffolds (BRS) promise to counteract late thrombosis by the absence of residual foreign material over time and the restoration of functional endothelial coverage. However, although currently available data are controversial, initial post-marketing studies have raised some concerns about the putative increased early thrombogenicity of BRS as compared to currently available second-generation DES4. Incidence rates, putative mechanisms and prevention strategies of scaffold thrombosis are discussed below.
Incidence of scaffold thrombosis
The incidence of BRS thrombosis is poorly understood. The designation of “scaffold thrombosis” (ScT) is itself debated, because late thrombosis occurring when the bioresorption process is ongoing (or completed) should probably be better characterised by alternative BRS-specific terminologies, such as “in-marker thrombosis” or “target lesion thrombosis”. The BRS Academic Research Consortium will solve these semantic conundrums and add uniformity to the BRS literature by the standardisation and adaptation of current definitions incorporating timing (i.e., acute, subacute, late) and diagnostic certainty (definite, probable, possible).
While the pivotal ABSORB studies claimed a 0% incidence of ScT at long-term follow-up, the largest post-marketing data set available to date documented a 2.1% cumulative incidence of definite/probable ScT at six months4, with the same rate already documented at 30 days in another relatively large registry including patients with ST-elevation myocardial infarction5. Indeed, a meta-analysis of 4,309 patients treated with the Absorb BRS (Abbott Vascular, Santa Clara, CA, USA) from Ishibashi et al has questioned whether the higher than expected rate of ScT observed in some registries, but not in others, may be explained by a preferential utilisation in patients with acute coronary syndromes (ACS)6. Obvious limitations of such pooled analyses stem from the available data: most of the pooled studies lack a comparator, the data were site-reported, and the outcomes were not adjudicated by an independent committee, nor by central angiographic and imaging core labs. Also, for the evaluation of ScT, six to 12 months are undoubtedly an insufficient time frame to capture the entire occurrence of the phenomenon, which can be underestimated. Finally, the studies included vary in terms of various considerations, including design, inclusion criteria and duration of follow-up. Taking these limitations into account, Ishibashi et al calculated a rate of definite/probable ScT of 0.8% at 30 days (Figure 1) and 1.2% at a weighted average follow-up duration of 10.3 months, with corresponding figures of 0.9% and 2.2% in stable and ACS patients, respectively.
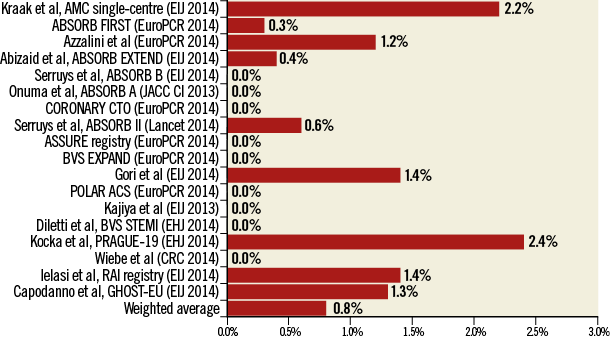
Figure 1. Early and total scaffold thrombosis in published and unpublished BRS series. EHJ: European Heart Journal; EIJ: EuroIntervention; JACC CI: JACC Cardiovascular Interventions. Adapted from Ishibashi et al5.
Pathophysiology of scaffold thrombosis
Assuming similar mechanisms of thrombosis for DES and BRS, different sets of multifactorial causes can be postulated (Figure 2). The unexpectedly high rates of early ScT noted in some studies (Figure 1) suggest that procedure-related factors may play an important role.
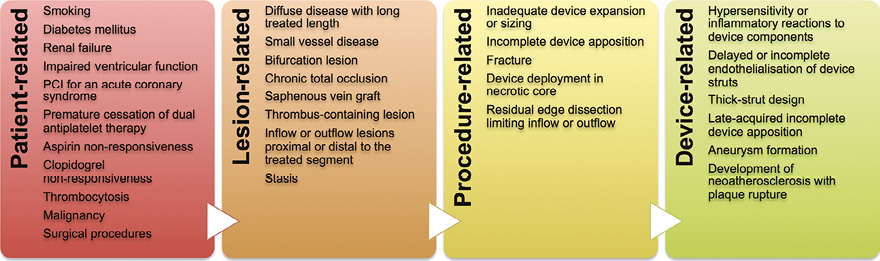
Figure 2. Putative mechanisms of scaffold thrombosis.
Patient-related factors
Individual predisposing conditions such as smoking, diabetes mellitus, chronic kidney disease, impaired left ventricular function and PCI in the context of an ACS have consistently been linked with thrombosis of DES, and a similar link with ScT, if confirmed, would not come as a surprise7. Thrombosis is a platelet-mediated process, which highlights the importance of additional platelet-specific risk factors, including thrombocytosis, clopidogrel non-responsiveness, and dual antiplatelet therapy (DAPT) discontinuation.
Lesion-related factors
DES thrombosis tends to occur more frequently in complex lesion subsets, such as diffuse coronary artery disease with long treated segments (a typical target of BRS therapy), small vessels, thrombus-containing lesions, saphenous vein grafts and bifurcations7. Significant angiographic predictors of ScT have not yet been identified, although it seems reasonable to assume that the challenges of the above-mentioned subsets remain (or may even be amplified) with BRS.
Procedure-related factors
Typically but not exclusively (Moving image 1-Moving image 3), early ScT is explained by procedure-related issues (i.e., fracture, acute incomplete apposition, underexpansion, flow-limiting dissection). Acute incomplete scaffold apposition has been reported at ~6% by optical coherence tomography, being observed more frequently in fibrocalcific plaques8. Lack of BRS strut apposition at baseline is related to the presence of uncovered struts and intraluminal masses at six months9. An early delay in strut coverage has been documented at the site of overlaps in preclinical models of the Absorb BRS compared with second-generation DES10.
Device-related factors
Activation of coagulation pathways in association with flow disturbances at bulky BRS struts may play a pivotal role in the pathophysiology of early ScT, consistent with seminal findings of prior clinical studies addressing the impact of stent design (Figure 3)11. The much greater dimension of BRS struts compared with DES might also provoke a substantial delay in re-endothelialisation12.
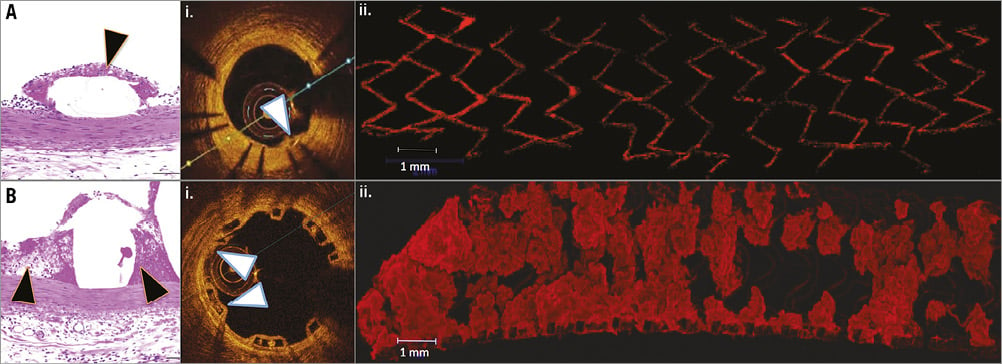
Figure 3. Comparison of acute thrombogenicity in contemporary thin-strut DES (A) and BRS (B) by standard histopathology (H&E stain), optical coherence tomography (OCT) (i) and confocal microscopy (ii). Notably, there is minimal deposition of fibrin-rich thrombus in the area around the DES struts (black arrowhead) (A) as compared to BRS (B). Similarly, OCT shows greater thrombus burden in the area around the stent struts in BRS as compared to DES (white arrowheads). Confocal microscopy after staining for platelet markers CD61/42b confirms the substantially greater presence.
Another potential trigger to ScT pertains to the inflammatory reactions observed acutely after implantation and chronically during the biodegradation phase13. Finally, cases of late-acquired incomplete strut apposition and neoatherosclerosis of BRS have been reported, two device-related phenomena which might also theoretically trigger late ScT14,15.
What about scaffold thrombosis in bifurcation lesions?
Historically, bifurcation PCI has been linked with a higher chance of stent thrombosis. The clinical relevance of DES thrombosis at this site is remarkable, being associated with a tenfold higher in-hospital mortality than stent thrombosis at a non-bifurcation site16.
The use of BRS in coronary bifurcations carries many limitations, including the uncertain outcome of procedural steps known to be important with DES but potentially hazardous with a breakable device, such as proximal optimisation, side branch fenestration and kissing balloon inflation.
Most techniques of double DES necessitate the placement of multiple metal layers; this “complex” final stent architecture impairs homogeneous metal strut endothelialisation and increases the risk of thrombosis. These issues can be significantly augmented by the use of thick-strut BRS, particularly with techniques entailing triple (i.e., internal crush) or double (i.e., culotte) strut layering. Until more data are available to provide guidance in this field, complex bifurcation scaffolding procedures should ideally be avoided with BRS, or techniques with least strut layering (i.e., T, T and small protrusion) preferred when a second scaffold (or stent) is needed.
Prevention of scaffold thrombosis
The instructions of the manufacturer should be followed thoroughly to minimise the chance of fracturing the BRS, or of leaving it underexpanded and/or incompletely apposed. These include meticulous procedural steps which deal with vessel sizing, lesion preparation, scaffold implantation, post-dilatation and the liberal use of intracoronary imaging to anticipate, detect and correct any possible technical shortcomings17. BRS overlaps should be limited and scaffold-to-scaffold techniques preferred when more than one BRS is needed. Caution is required in the use of BRS in ACS patients with large thrombus burden, where prasugrel or ticagrelor is the mainstay of therapy.
The optimal length of DAPT after BRS implantation has not been defined. Although recent European guidelines for myocardial revascularisation recommend DAPT for six months in most patients receiving DES, there is no BRS study supporting this recommendation, hence a more prudent approach with longer DAPT duration seems preferable at present. Indeed, in the ABSORB II trial, 83% of patients were on DAPT at one year, despite the study protocol allowing for a shorter six-month duration18. Similarly, in the GHOST-EU registry, DAPT was prescribed for 12 months in 94% of patients4. De-escalating strategies (i.e., prasugrel or ticagrelor for one month, clopidogrel thereafter) are used in some centres to minimise the risk of acute ScT and to best balance the risk of ischaemia and bleeding in the individual patient, although the net benefit of this strategy remains unproven at this stage. Continuation of DAPT beyond 12 months in patients at low bleeding risk could also be regarded as a viable strategy in view of recent data from a large randomised DES trial19. In patients treated with BRS, DAPT management in the context of non-cardiac surgery should be tailored in view of patient- and surgery-specific considerations.
Conclusions
The incidence of early ScT apparently resembles that of first-generation DES, with higher rates reported in ACS. Conversely, the incidence of late ScT is unknown. While the putative benefits of BRS are expected to accrue at late and very late term, clustering of thrombosis within the first 30 days in some studies suggests that procedure-related factors might play an important role. Along with the standardisation of implantation procedures, the refinement of contemporary BRS technologies (i.e., thinner struts with preserved radial strength) is warranted to reduce the risk of early ScT. Large BRS series are needed to identify predictors of early and late ScT and to provide guidance on prevention strategies and best management options at different follow-up intervals.
Conflict of interest statement
D. Capodanno reports receiving consulting fees or honoraria from Eli Lilly, Daiichi-Sankyo, AstraZeneca, and Abbott Vascular. The other authors have no conflicts of interest to declare.
Online data supplement
Moving image 1. Coronary angiography (cranial view) of a 55-year-old patient with diabetes mellitus presenting with angina symptoms and documented anterolateral ischaemia. The left anterior descending artery shows a long severe disease of the mid portion. Severe proximal and distal stenoses of a large diagonal branch are also observed.
Moving image 2. Final result after percutaneous coronary intervention of the left anterior descending artery with two bioresorbable vascular scaffolds (Abbott Vascular, Santa Clara, CA, USA) 3.0×28 mm, implanted with a scaffold-to-scaffold technique after extensive predilatation and post-dilated with a 3.0 non-compliant balloon at high pressure. Residual undilatable 20% stenosis of the distal scaffold was observed in the proximal portion.
Moving image 3. Cranial view after percutaneous coronary intervention of the proximal first diagonal with a 3.0×18 mm scaffold. The proximal scaffold implanted in the left anterior descending artery presents with diffuse haziness suggestive of acute thrombosis, confirmed by retrieval of thrombotic particulate after manual thrombectomy. Notably, the patient was not pre-treated with P2Y12 inhibitors, while the activated coagulation time was 350 seconds. Since optical coherence tomography was not performed, a mechanical reason cannot be excluded, although the instructions of the manufacturer were carefully followed at implantation and the final angiographic result was felt to be adequate (Moving image 2). As such, a clear cause for thrombosis could not be identified in this case, although several putative risk factors must be acknowledged, including diabetes, diffuse disease with long treated length, inadequate platelet inhibition and implantation of a device with thick-strut design. Conservative management with abciximab (bolus plus 12 hours infusion) on top of a ticagrelor loading dose was undertaken. No further dilatation was attempted in view of the evident thrombus resolution at control angiography with no chest pain and TIMI 3 flow. The in-hospital clinical course was uneventful and the patient was safely discharged with recommendation for 12 months dual antiplatelet therapy with aspirin and ticagrelor.
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 1. Coronary angiography (cranial view) of a 55-year-old patient with diabetes mellitus presenting with angina symptoms and documented anterolateral ischaemia. The left anterior descending artery shows a long severe disease of the mid portion. Severe proximal and distal stenoses of a large diagonal branch are also observed.
Moving image 2. Final result after percutaneous coronary intervention of the left anterior descending artery with two bioresorbable vascular scaffolds (Abbott Vascular, Santa Clara, CA, USA) 3.0×28 mm, implanted with a scaffold-to-scaffold technique after extensive predilatation and post-dilated with a 3.0 non-compliant balloon at high pressure. Residual undilatable 20% stenosis of the distal scaffold was observed in the proximal portion.
Moving image 3. Cranial view after percutaneous coronary intervention of the proximal first diagonal with a 3.0×18 mm scaffold. The proximal scaffold implanted in the left anterior descending artery presents with diffuse haziness suggestive of acute thrombosis, confirmed by retrieval of thrombotic particulate after manual thrombectomy. Notably, the patient was not pre-treated with P2Y12 inhibitors, while the activated coagulation time was 350 seconds. Since optical coherence tomography was not performed, a mechanical reason cannot be excluded, although the instructions of the manufacturer were carefully followed at implantation and the final angiographic result was felt to be adequate ().