
Abstract
Aims: The aim of this study was to report on the midterm outcomes of patients undergoing percutaneous coronary intervention with bioresorbable vascular scaffolds (BVS) for the treatment of acute coronary syndromes (ACS) and compare with those of patients with stable coronary artery disease (sCAD).
Methods and results: One thousand four hundred and seventy-seven (1,477) patients underwent implantation of one or more BVS (Absorb BVS; Abbott Vascular, Santa Clara, CA, USA) at 11 European centres and were included in the GHOST-EU registry. Admissions comprised 47.1% of the patients (951 BVS) with ACS, and 52.8% (1,274 BVS) with sCAD. During a median follow-up of 384 (359-460) days, patient-oriented endpoints (PoCE), including all-cause death, any infarction, any revascularisation, were recorded in 271 patients (12-month incidence in ACS patients: 18.5% vs. 11.6% in the sCAD group, p<0.001). Device-oriented composite endpoints (DoCE), cardiac death, target vessel infarction and target lesion revascularisation, were observed in 98 patients (12-month incidence of 4.2% in the sCAD group, 6.4% in the ACS group; p=0.052). The 12-month incidence of definite scaffold thrombosis was 2.6% in ACS patients and 0.8% in XIENCE patients (p=0.006). In multivariate analysis, ACS was a predictor of DoCE (HR: 2.26 [1.34-3.81], p=0.002), PoCE (HR: 1.71 [1.13-2.58], p=0.011), and stent thrombosis (HR: 2.51 [1.13-5.60], p=0.025). In contrast, the incidence of target lesion revascularisation was not different between groups. There was no difference in the incidence of any of these endpoints among the different clinical presentations (unstable angina, non-ST-elevation infarction and ST-elevation infarction).
Conclusions: PoCE, DoCE and scaffold thromboses were more frequent in ACS patients, without any difference among different forms of ACS.
Abbreviations
BVS: bioresorbable vascular scaffold(s)
DES: drug-eluting stent(s)
MI: myocardial infarction
ScT: scaffold thrombosis
TLF: target lesion failure
TLR: target lesion revascularisation
TVF: target vessel failure
TVR: target vessel revascularisation
Introduction
Bioresorbable vascular scaffolds (BVS) were introduced in the hope of addressing some of the late complications associated with implantation of metal stents. Particularly, late lumen enlargement, the restoration of the vasomotor properties of the coronary artery and the “sealing” of thrombotic plaques represent attractive opportunities for patients with acute coronary syndromes (ACS)1,2. Experiences reported to date, however, focus particularly on ST-elevation infarction patients; data on other forms of ACS are less well represented3-6. Further, reports available to date lack a control group without ACS (stable coronary artery disease [sCAD]). Clinical data from the international and multicentre GHOST-EU registry were recently published7. Reflecting clinical routine in tertiary centres, approximately 50% of the patients enrolled in this registry had a diagnosis of ACS as defined by unstable angina (uAP), non-ST-elevation myocardial infarction (NSTEMI) and ST-elevation myocardial infarction (STEMI). We report on the early and midterm outcomes of percutaneous intervention with BVS in these patients and compare them with those recorded in patients with sCAD.
Methods
PATIENT POPULATION AND PROCEDURES
The GHOST-EU registry is a retrospective, multicentre registry conducted at 11 European centres. All patients undergoing intervention with the everolimus-eluting BVS (Absorb; Abbott Vascular, Santa Clara, CA, USA) between November 2011 and June 2014 were eligible for recruitment. Further details on the structure of the registry can be found in our previous article7.
OBJECTIVES
The primary aim of this study was to test whether ACS at index procedure was an independent predictor of patient-oriented composite endpoints (PoCE) and device-oriented composite endpoints (DoCE). An analysis was also performed for scaffold thrombosis. Major adverse cardiovascular events (MACE) were defined as the composite of cardiovascular death, any myocardial infarction (MI), and clinically driven target lesion revascularisation (TLR). PoCE were the composite of all-cause death, any myocardial infarction (MI) and any repeat revascularisation. DoCE were the combination of cardiac death, target vessel myocardial infarction and clinically driven target lesion revascularisation (TLR).
PROCEDURES AND FOLLOW-UP
Interventions were performed as previously described7. Follow-up data were collected during clinical visit and/or by direct telephone contact. When necessary, multiple attempts were made to contact patients by telephone, in writing and/or through the family physician. Patients for whom a 12-month follow-up was not available were censored at the time of last contact. Quantitative coronary analysis was performed on-site by experienced personnel using methods previously described8.
Scaffold thrombosis was classified according to the Academic Research Consortium criteria9. Other definitions are reported in detail in previous papers from our group7,8.
STATISTICAL ANALYSIS
Data for continuous variables were tested for normality by inspection of the Q-Q plots, and are correspondingly presented as mean±standard deviation or median (interquartile range) and compared using parametric or non-parametric tests. Categorical variables are presented as counts and percentages, and were compared using the Fisher’s exact test. In order to assess differences of lesion-based parameters between groups, where multiple measurements on one patient may occur, we fitted linear mixed models (quantitative variables) and generalised linear mixed models (categorical variables) and tested whether there was a group effect. Unadjusted event rates during follow-up were estimated by the Kaplan-Meier method and compared by the log-rank test. In case of competing risks, cumulative incidence functions were computed. Cox proportional hazard models were used to obtain estimates for hazard ratios associated with potential explanatory variables (Table 1, Table 2). Parameters associated with a p<0.1 in univariate analysis were entered in a multivariable model. P-values <0.05 were considered significant. Since the 12-month follow-up was performed by protocol, the Cox regression analysis was performed for this time point. Statistical analyses were performed using MedCalc (MedCalc Software, Mariakerke, Belgium).
Results
PATIENT POPULATION
Between November 2011 and June 2014, a total of 2,216 BVS were implanted in 1,477 patients; 47.1% (697) of the patients (951 BVS) were admitted with ACS, 52.8% (780, 1,274 BVS) with stable CAD. In the ACS group, 248 (35.5%) were admitted with STEMI, 259 (37.1%) with NSTEMI and 190 (27.3%) with unstable AP.
PROCEDURAL DETAILS AND MEDICATIONS
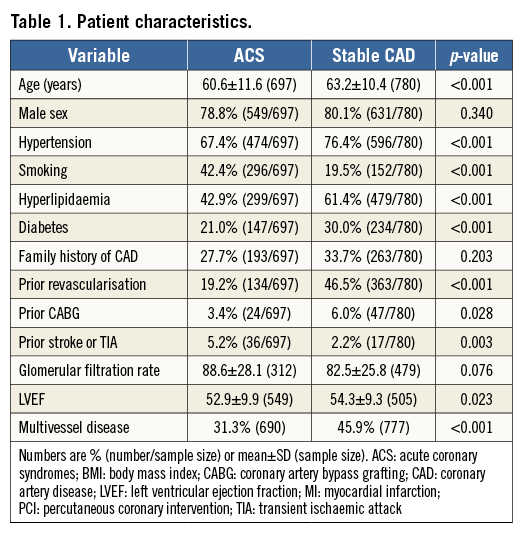
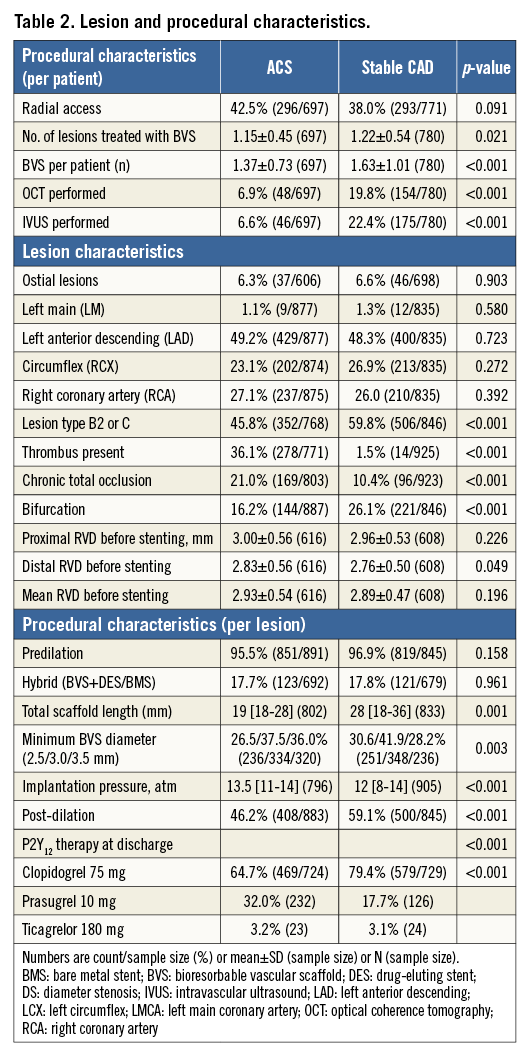
Clinical, lesion and procedural characteristics are presented in Table 1 and Table 2. Supplementary Table 1 and Supplementary Table 2 report details of the patient and lesion characteristics according to presentation type (unstable angina, NSTEMI or STEMI) within the ACS group. There were major differences between groups in most clinical parameters (Table 1, Table 2). Age (60.6±11.6 vs. 63.2±10.4 years, p<0.001), the prevalence of hyperlipidaemia, hypertension, diabetes, and of prior revascularisations or stroke was higher in the sCAD group, while a smoking history was more frequent in ACS patients (all p<0.0001). Except for coronary thrombus, characteristics of lesion complexity, including bifurcation lesions and chronic total occlusions, were more frequent in the sCAD group (all p<0.0001). The total implanted scaffold length was shorter (19 [18-28] vs. 28 [18-36] mm, p=0.0001) and scaffolds tended to be larger (3.5 mm BVS used: 36% vs. 28.2%) in patients with ACS. Predilation was performed in most lesions in both groups (95.5% vs. 96.9%, p=0.158); post-dilation was more often performed in the sCAD group (46.2% vs. 59.1%, p<0.001). Intracoronary imaging was less frequently performed in patients with ACS (optical coherence tomography: 19.5% vs. 7.0%; p<0.001, intravascular ultrasound: 23.3% vs. 6.4%; p<0.001). Antiplatelet therapy with prasugrel or ticagrelor was more often prescribed in the ACS group.
CLINICAL OUTCOMES
The median follow-up was 365 (359-365) days. Twelve-month follow-up data were available in 1,207 (82%) patients. The incidence of intraprocedural complications was similar between groups (Table 3). Event rates during follow-up are described in Table 4, Table 5, Figure 1A, Figure 1B and Figure 2. At 12 months, the incidence of MACE (49 [7.6%] vs. 39 [4.6%], p=0.016) and PoCE (n=123 [18.5%] vs. 83 [11.6%], p<0.001) was higher in the ACS group, while that of DoCE (42 [6.4%] vs. 30 [4.2%], p=0.052) and of the single endpoints showed a trend in the same direction. Also, the incidence of both definite and definite+probable scaffold thrombosis (ScT) (n=18 [2.6%] vs. 6 [0.8%], p=0.006 and 19 [2.8%] vs. 10 [1.4%], p=0.047) (Table 5) was higher in the ACS group.
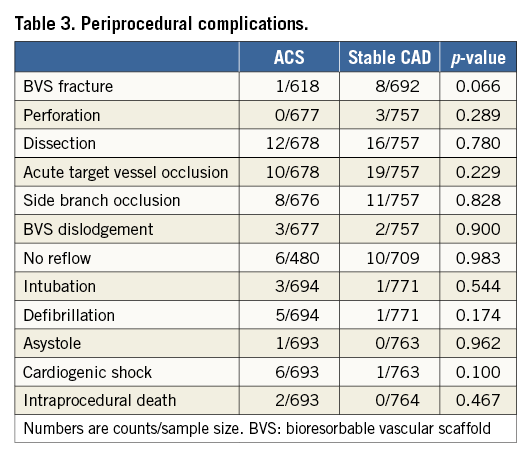
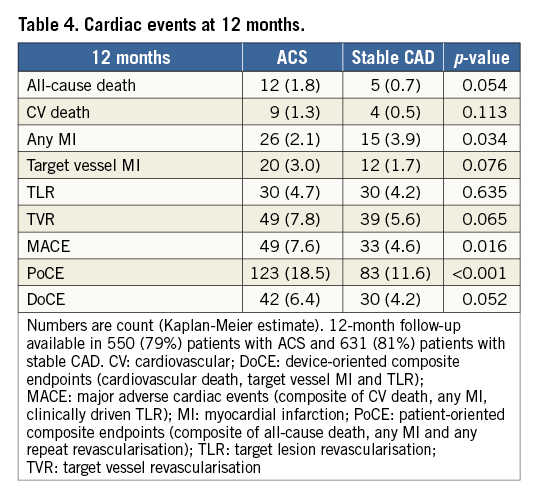
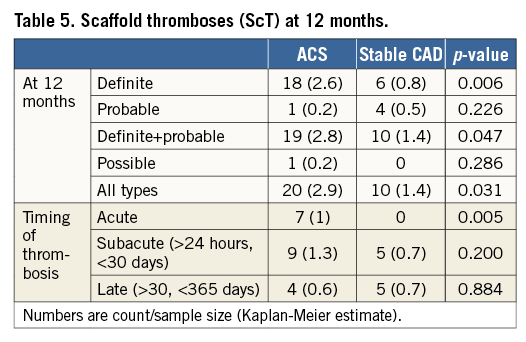
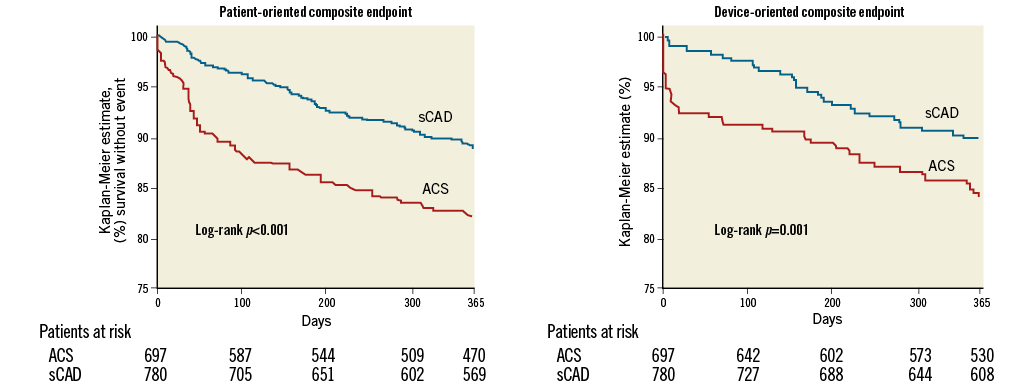
Figure 1. The incidence of device-oriented and patient-oriented composite endpoints.
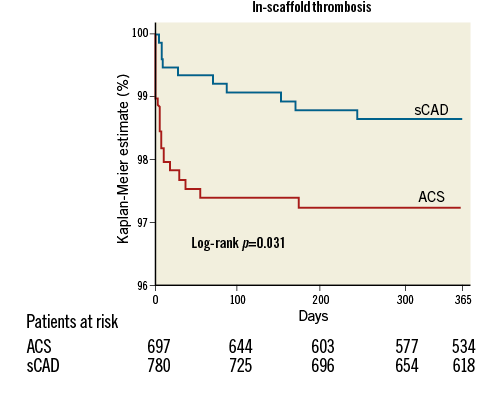
Figure 2. The incidence of scaffold thrombosis (definite, probable and possible).
The type of ACS (unstable angina, NSTEMI or STEMI) had no impact on the incidence of these events (Figure 3), even though a slightly lower incidence of PoCE was observed in unstable angina patients.
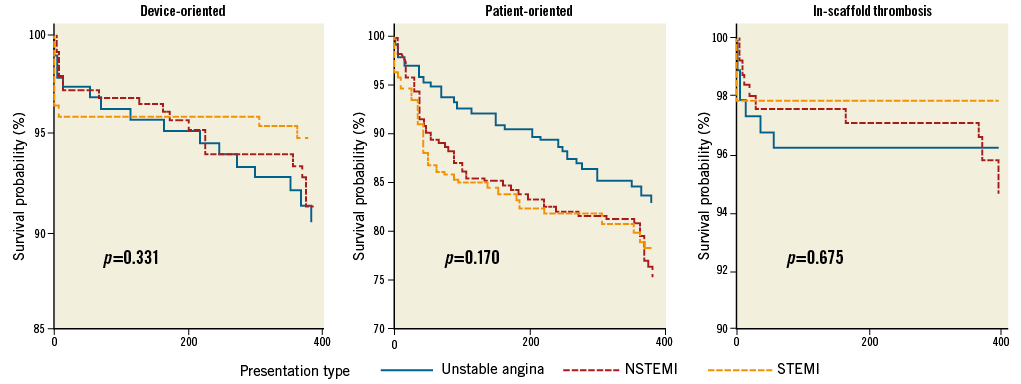
Figure 3. The incidence of the three endpoints by presentation type.
PREDICTORS OF EVENTS AND THE ROLE OF IMPLANTATION STRATEGY IN ACS
The list of the variables considered for this analysis along with the corresponding HRs and p-values are presented in Supplementary Table 3-Supplementary Table 5. ACS at implantation was an independent predictor of 12-month DoCE (p=0.002, HR: 2.26 [1.34-3.81]), PoCE (p=0.011, HR: 1.71 [1.13-2.58]), and stent thrombosis (p=0.025, HR: 2.51 [1.13-5.60]). In patients with ACS, implantation of BVS with a nominal diameter at least 10% larger than the reference vessel diameter was associated with PoCE (27% vs. 11.2%, p<0.001, HR: 2.65 [1.65-4.32]), DoCE (9% vs. 4.6%, p=0.044, HR: 2.28 [1.03-5.08]) and ScT (6.2% vs. 0.9%, p=0.011, HR: 7.80 [1.63-37.43]). Also, reference vessel diameter (RVD) (data available in n=1,227 lesions, 1,020 patients, 465 with ACS) was a predictor of PoCE (p<0.001, HR: 0.47 [0.33-0.66]), DoCE (p=0.023, HR: 0.51 [0.28-0.91] and ScT (p=0.025, HR: 0.40 [0.18-0.89]). In contrast, the choice of a BVS with a nominal diameter smaller than the RVD (“undersizing”) was not associated with any of the endpoints (PoCE: p=0.53, HR: 0.82 [0.45-1.51], DoCE: p=0.83, HR: 1.11 [0.44-2.78], and ScT: p=0.92, HR: 0.92 [0.19-4.40]). Post-dilation alone had no impact on DoCE (p=0.656, HR: 0.86 [0.47-1.60]), PoCE (p=0.779, HR: 0.95 [0.67-1.36]) or ScT (p=0.58, HR: 0.80 [0.37-1.76]).
All these associations remained valid also when only STEMI patients were included.
Discussion
SUMMARY OF THE RESULTS
In the present report, ACS was an independent predictor of both DoCE and PoCE. These results are not surprising: the incidence of events in our BVS group was in line with that reported in previous drug-eluting stent studies10,11. Similarly, while no difference was observed in terms of TLR, BVS implantation in the setting of ACS was also associated with a higher incidence of ScT. Importantly, no difference was observed in terms of DoCE and ScT among patients admitted for unstable angina, NSTEMI or STEMI.
In line with recently reported data12,13, a higher incidence of events was observed in patients in whom a BVS at least 10% larger than the target vessel was implanted. Beyond the risk of vessel trauma and edge dissection associated with oversizing, there are additional considerations that are specific to BVS to explain this observation12,14. Due to the larger strut width, the implantation of BVS results in a larger strut to artery ratio (footprint) as compared to DES. As a consequence, the risk of underexpansion is higher than with DES. Particularly when associated with inaccurate lesion preparation (no predilation or predilation with small balloons as often performed in the treatment of ACS lesions), this may ultimately lead to decreased luminal flow area and increased inter-strut flow turbulence. In analogy, small RVDs were a predictor of all event types, a finding that was also observed in the ABSORB III study13. These data highlight the importance of target vessel size and scaffold deployment also in the setting of ACS. Further supporting this important concept, while ACS at index was an important determinant of outcomes in the overall population in our recently published report, this association did not reach significance when BVS were implanted using a technique specifically aimed at improving BVS expansion12. Also, in the current analysis, ACS was not a predictor of device endpoints in patients in whom post-dilation was performed. While post-dilation might potentially represent a risk factor for no reflow in thrombotic lesions of patients with ACS, and while post-dilation as a single parameter was not a predictor of events in our database (Supplementary Table 3-Supplementary Table 5), the fact that this manoeuvre is part of the optimal BVS implantation strategy is recognised15.
BVS IN ACS
While they provide effective mechanical revascularisation of culprit lesions, permanent metallic stents also have important drawbacks, and ACS represents a setting in which bioresorbable technologies might provide important advantages. Profound differences in plaque biology and pathophysiology exist between sCAD and ACS, which has profound implications with regard to the goal of revascularisation. In addition to the acute improvement of coronary haemodynamics and resolution of the stenosis, plaque stabilisation assumes a central role in ACS, and the inflammatory/oxidative reactions associated with permanent implantation of metal stents might represent a disadvantage of traditional stents16-22. In contrast, recent intracoronary imaging data of ACS patients treated with BVS described the formation of a neointimal layer 12 months after implantation6,23-27 associated with an increase in (stable) fibrous and fibro-fatty content and a decrease in features of unstable necrotic core and dense calcium tissue28. Along with the mechanical resolution of the stenosis, it has been proposed that this layer, with characteristics compatible with those of fibrous tissue, might prevent repeat rupture/ulceration, reducing the risk of intracoronary thrombosis24. As such, a biological rationale exists to suggest that permanent implants might also not be necessary in ACS patients.
To date, data on the outcome after BVS treatment of ACS are available but remain limited. With regard to acute and short-term procedural outcomes, data from the POLAR-ACS, the Polish National Registry (about 50% of the patients with ACS), the GHOST-EU (47% ACS) and AMC PCI registries (39% ACS), as well as STEMI-specific registries29-31 have documented the safety of BVS implantation4,7,32-34. At six months, admission for ACS was not a predictor of outcomes in the GHOST-EU database, but, similar to the Mainz registry4, an unexpectedly high incidence of ScT was reported12. Similarly, ACS at index did not influence outcomes in the ABSORB III trial13. In the BVS-EXAMINATION trial, a propensity score-based comparison of Absorb BVS and XIENCE drug-eluting stents (Abbott Vascular) in STEMI, the DoCE rate was similar between groups, but a numerically higher incidence of ScT was recorded in the BVS group2.
Limitations
Patients with ACS differed in many parameters from those without ACS. Even though the Cox regression analysis was adjusted for these factors, and even though it has already been shown that parameters like diabetes do not influence the prognosis of patients treated with BVS35, interactions may be complex and difficult to correct for. This registry was not a randomised, controlled trial, there was no external monitoring or event adjudication, and the follow-up in the whole population was not possible. Also, patients were treated at a time when the importance of the implantation technique remained to be demonstrated. As a consequence, the present data reflect early BVS experiences. A recent paper from our group demonstrates that the use of a BVS-specific implantation strategy is associated with a marked reduction in events, offsetting the risk associated with an unstable clinical presentation12. Although these limitations are to be acknowledged, this registry provides real-life data, demonstrating the importance of careful lesion preparation, vessel sizing, and BVS expansion. Further, QCA data were not analysed centrally and, due to logistical reasons, data from some of the centres were not available. However, the subgroup of patients in which these data were available did not differ from the overall study cohort with respect to both clinical/procedural parameters and outcomes. Finally, ACS patients are not a homogeneous population and important differences exist between presentation types. Whether these differences also result in differences in the predictors of events will need to be investigated in future studies.
Conclusions
We report 12-month follow-up data from the early experience with BVS in the setting of ACS. In line with previous data from metal stents36, a history of ACS was associated with an increased incidence of DoCE, PoCE and ScT, but not TLR. Oversizing, and not undersizing, appeared to be a determinant of events. These observations offer further information on the importance of the deployment technique in this setting.
| Impact on daily practice In patients treated with bioresorbable scaffolds, a history of acute coronary syndrome at index procedure was a predictor of device-oriented and patient-oriented endpoints, including scaffold thrombosis. As for stable patients, relative oversizing at the time of implantation appeared to be a determinant of events. |
Conflict of interest statement
Supplementary data
Supplementary Table 1. Patient characteristics.
Supplementary Table 2. Lesion and procedural characteristics.
Supplementary Table 3. Predictors of DoCE at 12 months.
Supplementary Table 4. Predictors of PoCE at 12 months.
Supplementary Table 5. Predictors of ScT at 12 months.

