Abstract
Aims: In this multicentre prospective registry we sought to evaluate the immediate and midterm clinical outcomes following single or multiple overlapping bioresorbable vascular scaffold (BVS) implantation in the STEMI setting.
Methods and results: A prospective cohort analysis was performed on all STEMI patients who underwent primary PCI with BVS implantation. Between December 2012 and February 2014, 1,232 STEMI patients underwent primary PCI at the participating centres. Of these, 74 (6.0%) received a BVS, 18 (24.3%) of them were multiple and overlapping. Procedural success was obtained in 72 (97.3%) cases without differences between the groups (overlapping BVS 100% vs. single BVS 96.4%, p=0.5). One patient experienced a reinfarction due to subacute BVS thrombosis which was successfully managed with balloon-only PCI while the other patient had a “slow-flow” phenomenon (final TIMI flow 2). At six-month follow-up, two non-fatal MI (2.7%), three target lesion revascularisations (4.1%), and one subacute BVS thrombosis were reported in three patients (one [5.6%] overlapping BVS and two [3.6%] in the single BVS group, p=0.5). All the events were successfully managed with re-PCI.
Conclusions: BVS implantation in STEMI patients can be successfully performed with a high procedural success rate and encouraging midterm outcomes. Larger randomised trials and longer follow-up are needed to assess the potential clinical benefit of BVS versus new-generation DES in this setting.
Introduction
Primary percutaneous coronary intervention (PPCI) represents the therapy of choice in the setting of ST-segment elevation myocardial infarction (STEMI) when it can be performed in a timely fashion by an experienced team1,2. Refinement of interventional techniques, optimisation of antiplatelet and antithrombotic therapy, and the evolution from bare metal stents (BMS) to first-generation and now new-generation drug-eluting stents (DES) have significantly improved the outcomes of STEMI patients3-6. New data suggest that newer-generation DES (particularly cobalt-chromium [Co-Cr] everolimus-eluting stents [EES]) may be considered the standard of care for the treatment of STEMI patients undergoing PPCI7,8.
However, the implantation of a permanent device in the vessel wall can be associated with important limitations, such as durable caging of the vessel with consequent permanent impairment of the vasomotion, side branch jailing, impossibility of late lumen enlargement, non-invasive imaging and future surgical revascularisations9. The everolimus-eluting bioresorbable vascular scaffold (BVS) (Absorb; Abbott Vascular, Santa Clara, CA, USA) has been designed initially to accomplish the same goals as metallic platform DES (seal dissections, prevent acute recoil, and inhibit neointimal hyperplasia) and then to overcome their limitations, disappearing entirely within three years and restoring native pristine vessel state. BVS have been shown to be safe and effective in the treatment of simple lesions of patients with stable coronary artery disease10-13. However, to this day limited data are available on the use of this new technology in patients with acute coronary syndromes (ACS) and particularly in STEMI lesions14,15. The aim of this registry was to assess the feasibility and immediate and midterm outcomes following implantation of single or multiple overlapping BVS during PPCI.
Methods
The RAI (“Registro ABSORB Italiano”) registry is an ongoing, spontaneous, multicentre prospective data collection on patients undergoing unrestricted (including bifurcations, long lesions, in-stent restenosis, chronic total occlusions, ACS) second-generation (1.1) everolimus-eluting BVS implantation at different Italian hospitals. This registry has been developed in cooperation with the “Mario Negri Sud” Research Institute, S. Maria Imbaro, Italy. The main characteristics of this device have already been described elsewhere16,17. The protocol of this registry was approved by the local ethical committee at each centre. The study was conducted according to the Declaration of Helsinki, and written informed consent was obtained from all study patients. As part of this ongoing prospective data collection, a cohort analysis was performed on all STEMI patients with symptom onset <24 hours from hospital admission who underwent PPCI with BVS implantation. The decision to implant a BVS rather than a stent was left to the operator’s discretion in the presence of suitable anatomy (absence of tortuosity and/or severe calcification proximal to the culprit lesion) and lesion (infarct artery reference diameter visually assessed at the culprit site ≥2.3 mm and ≤3.7 mm) characteristics, and in the absence of severe comorbidities (known at the time of hospital admission) with poor life expectancy. Intracoronary thrombus was angiographically identified and scored in five grades as previously described18.
An angiographic exclusion criterion was the presence of a stent thrombosis (ST) as a culprit lesion. On the other hand, lesion length superior to 28 mm was not an exclusion criterion, and implantation of BVS in overlap was allowed both in cases of long lesions and for bail-out stenting of edge dissections or insufficient lesion coverage. The overlap strategy (“marker-to-marker” or “marker-over-marker”) was left to the operator’s discretion, as were arterial access and periprocedural antithrombotics. All patients received a loading dose of antiplatelet drugs (500 mg aspirin i.v. and clopidogrel 600 mg or ticagrelor 180 mg or prasugrel 60 mg orally) periprocedurally. The dual antiplatelet therapy (DAT) regimen at discharge was with aspirin (100 mg daily) recommended indefinitely in association with clopidogrel or ticagrelor or prasugrel for 12 months. Intracoronary imaging, thrombus aspiration, lesion predilatation preceding implantation and post-dilatation (with a maximum diameter 0.5 mm greater than the BVS diameter) after BVS implantation were not mandatory but left to the operator’s discretion.
The primary endpoint of the study was procedural success, defined as BVS implantation at the “culprit” lesion site with less than 30% final stenosis and TIMI 3 flow without in-hospital major adverse cardiovascular events (MACE: cardiac death, myocardial infarction [MI] or need for emergent revascularisation). Furthermore, we evaluated the occurrence of cardiac death, MI, target lesion revascularisation (TLR) and BVS thrombosis at follow-up. BVS restenosis was defined as a diameter stenosis ≥50% within the scaffolded segment or 5 mm proximal or distal to the scaffolded segment19. Clinical events were defined according to the Academic Research Consortium definitions20. Clinical data were collected by hospital visit or telephone contact at two-month intervals. Angiographic follow-up was not scheduled but performed only in case of planned “step” revascularisation or if clinically indicated. All data provided by each site were anonymised, centrally collected, and assessed for quality. Source verification and queries generation from the coordinating centre to the participating sites were undertaken to account partly for the unavoidable bias of site-reported events adjudication.
Statistical analysis
Continuous variables were expressed as mean±SD. Comparisons of clinical, angiographic or procedure-related characteristics of patients were performed by means of Student’s t-test or Wilcoxon rank-sum test (continuous variables), or chi-square test (categorical), and on the basis of the distribution according to the presence of BVS overlapping or not. All analyses were conducted using SPSS software version 16.0 for Windows (SPSS Inc., Chicago, IL, USA), and all reported p-values are two-sided. Values of p<0.05 were regarded as statistically significant.
Results
At the participating centres, between December 2012 (when the first STEMI patient was enrolled in the RAI registry) and February 2014, 1,232 STEMI patients underwent PPCI with at least one stent implantation. Among these patients, 795 (64.5%) received DES, 363 (29.5%) received BMS and 74 (6.0%) BVS. Among the BVS patients, 56 (75.7%) received a single BVS, while 18 (24.3%) received at least two overlapping BVS, performed as intention to treat. Baseline clinical characteristics of the population according to the number of BVS implanted (single vs. overlapping) are summarised in Table 1.
Baseline lesion and procedural characteristics according to the number of BVS implanted are shown in Table 2. For illustrative purposes, Figure 1 and Figure 2 show OCT images following BVS implantation.
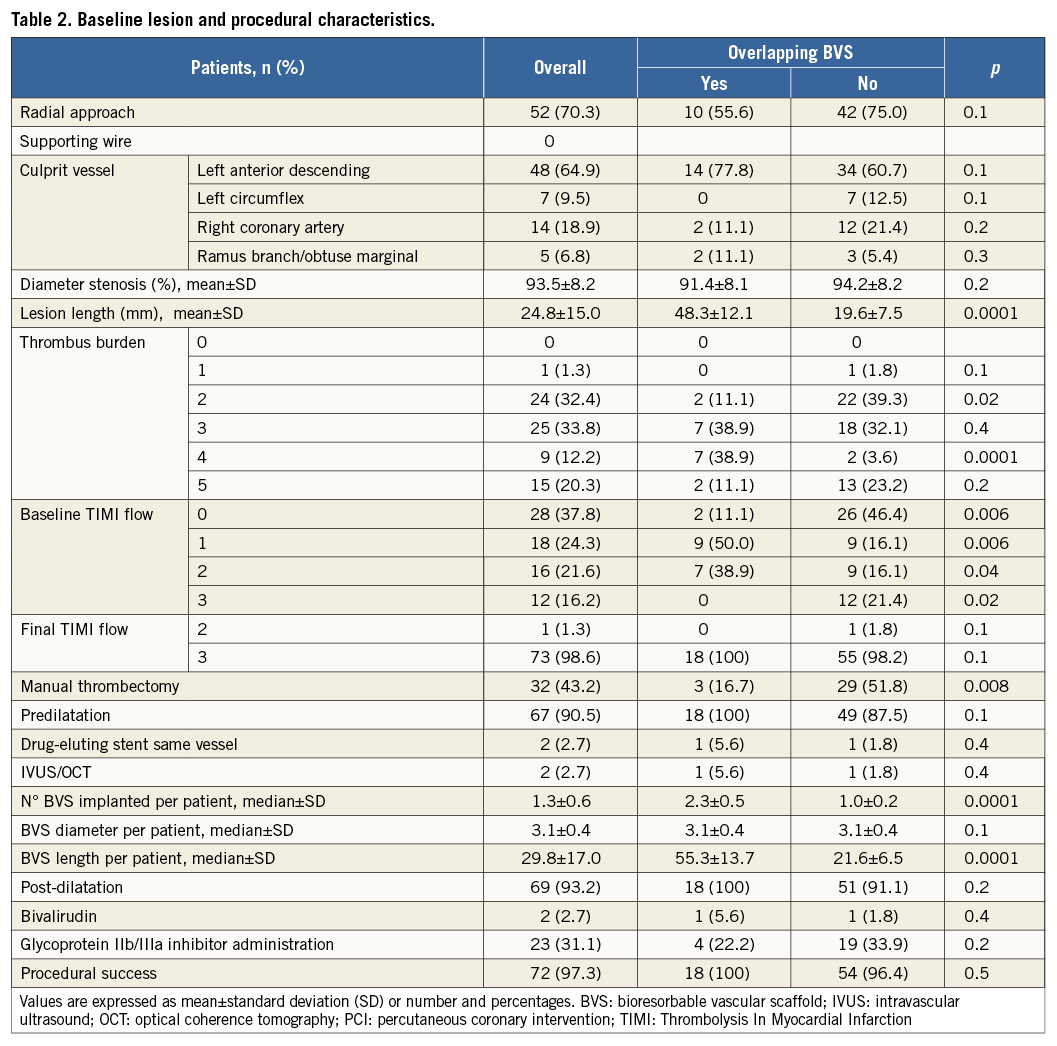
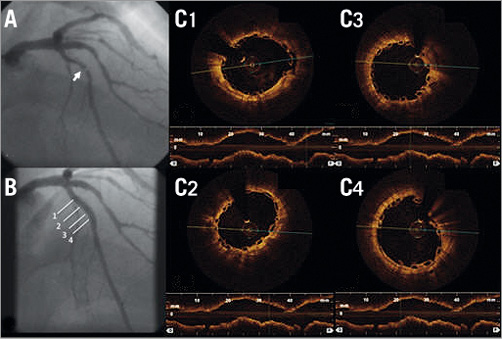
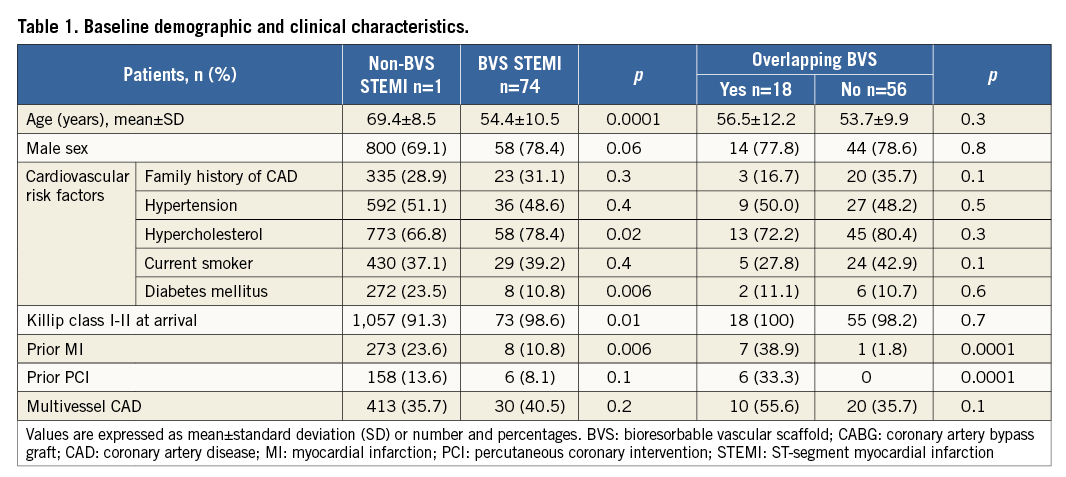
Figure 1. Coronary angiography and serial OCT images following BVS implantation of LAD. A) Coronary angiography showing critical stenosis of proximal LAD with thrombus (white arrow). B) Final angiographic result after thrombus aspiration, predilatation and BVS implantation. C) 1 to 4 - OCT frames showing good BVS strut apposition (1: proximal, 4: distal). White lines indicate the sites corresponding to the cross-sectional images.
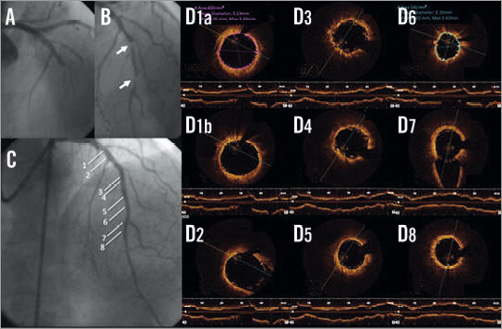
Figure 2. Coronary angiography and serial OCT images following BVS implantation of LAD. A) Coronary angiography showing a critical stenosis and a total LAD occlusion. B) Vessel recanalisation after passing the guidewire, with evidence of two critical stenoses (white arrows). C) Final angiographic result after implantation of two BVS. D1-D8) OCT frames showing good BVS strut apposition. White lines in panel C indicate the sites corresponding to the cross-sectional images (1a: BVS minimal lumen area at the proximal edge, 1b: BVS apposition at the proximal edge; 2,7: bifurcation sites; 3-4: overlapping segment; 8: distal).
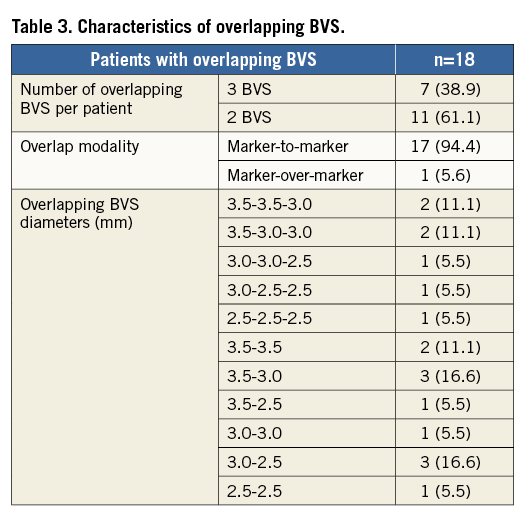
Among the patients who required BVS overlap, 11 (61.1%) received two BVS while seven (38.9%) received three. Most of the overlaps (14 [77.8%]) were performed in mid to distal LAD using the “marker-to-marker” strategy in order to minimise the percentage of vessel wall area covered by BVS struts. The characteristics of overlapping BVS are shown in Table 3.
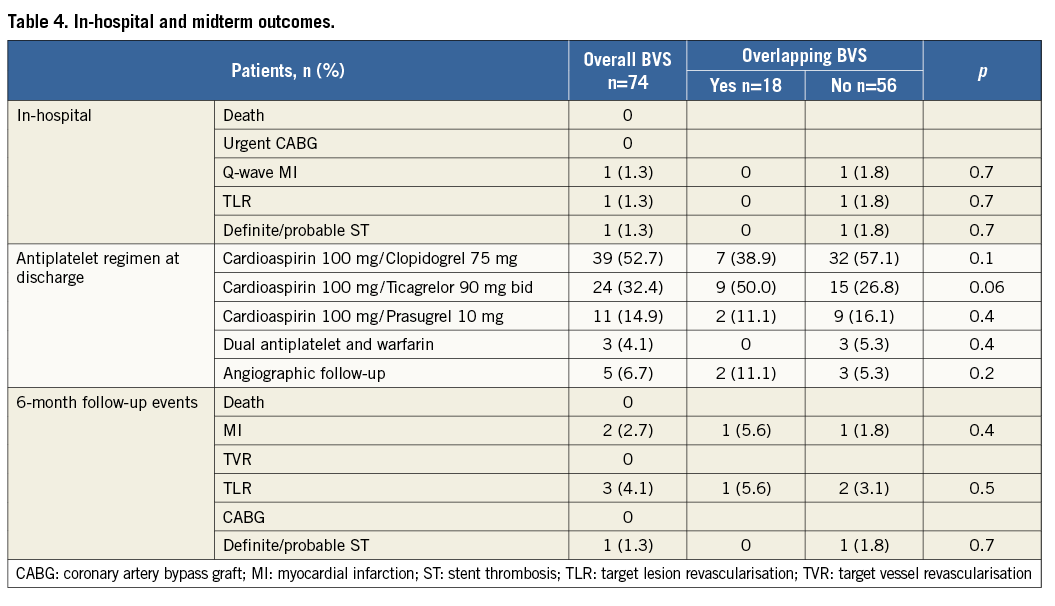
Procedural success was obtained in 72 (97.3%) cases without differences between the groups. One patient with anterior STEMI due to complete occlusion of mid LAD treated with single (3.5×18 mm) BVS implantation had a “slow-flow” phenomenon with final TIMI flow 2. The other patient experienced a subacute BVS thrombosis five days after an inferior STEMI treated with PPCI and single (2.5×18 mm) BVS implantation. This event occurred while the patient was on DAT (aspirin and clopidogrel) and was successfully managed by re-PPCI with non-compliant (NC) balloon inflation. Both patients survived and were discharged a few days later.
Among the 74 STEMI patients treated with BVS, 68 (91.9%) had a complete six-month follow-up while all had a five-month follow-up. In-hospital and midterm outcomes according to the number of BVS implanted are shown in Table 4. No cardiac death occurred, while two (2.7%) MI, three (4.1%) TLR, and one (1.3%) BVS thrombosis were reported in three patients (two single BVS [3.6%] and one overlapping BVS [5.6%], p=0.5). One patient, despite DAT treatment, had a recurrent inferior STEMI due to total occlusion of a single BVS implanted in the distal RCA 18 days after the index procedure. IVUS evaluation showed a short BVS malapposition due to BVS underdeployment (media-to-media diameter 4-4.5 mm) treated by NC balloon inflation followed by BMS implantation. The patient survived the event. In another patient undergoing non-culprit PCI, OCT analysis of two overlapping BVS previously implanted on the LAD showed focal in-BVS restenosis (85% diameter stenosis in the 3.0×28 mm BVS) successfully treated with an NC (3.5×15 mm at 22 atm) balloon inflation. The third patient underwent angiographic follow-up after six months because of recurrent angina. A focal restenosis at the proximal edge was found and treated with the implantation of another 3.5×12 mm BVS. The patient remained asymptomatic and the vessel patent at six-month angiographic follow-up.
Discussion
This multicentre registry of 74 STEMI patients treated with BVS implantation shows how this device can be considered as an interesting choice during PPCI. We observed a very high procedural success rate and low MACE both in-hospital and at six-month follow-up. Interestingly, we investigated the role of multiple overlapping BVS implanted during PPCI, and observed how this strategy had a similar outcome compared to single BVS implantation.
Two recent meta-analyses of patients treated with PPCI showed that the implantation of new-generation DES (especially a Co-Cr EES) was associated with significantly lower rates of TLR, cardiac death/MI, and definite/probable ST if compared to BMS and first-generation DES. On this basis, new-generation EES should be considered the first choice during PPCI7,8. However, some drawbacks of metallic platform DES might yield worse consequences in this setting. Firstly, they may hamper the physiological reactivation of vasomotion after successful PCI. Secondly, thrombus sequestration behind the struts (with late dissolution) and the vasoconstriction typical of the acute phase of STEMI may favour stent underdeployment and late malapposition, conditions associated with ST21,22. These limitations could be overcome by BVS, which have been shown to be associated with late vessel enlargement up to three-year follow-up23. Furthermore, theoretical advantages of BVS implantation during STEMI may be related to the younger age of typical STEMI patients, who may live many years after successful PPCI, thus deriving a benefit of not having a permanent metallic prosthesis in their coronary arteries.
Our results should be interpreted along with other papers investigating the role of BVS in similar settings. Kajiya and Wiebe first reported the feasibility of BVS implantation during PPCI in small single-centre populations of 25 and 11 STEMI patients, respectively24,25. Gori reported in-hospital and one-month outcomes of a consecutive series of patients treated with BVS implanted for acute coronary syndromes (n=66 STEMI, n=60 NSTEMI, and n=24 unstable angina) at a single centre. The authors showed similar outcomes with BVS compared to a matched population treated with the new-generation EES in the same setting. BVS thrombosis occurred in four out of 150 patients (2.6%) without differences compared with the control group (p=1)26.
Two recent larger studies investigated the acute clinical BVS performance in the STEMI setting14,15. Kocka reported the results of the prospective multicentre Prague 19 registry that enrolled 41 patients treated with BVS during PPCI. In this study, despite the absence of a specified clinical follow-up, the BVS yielded a very high success rate (98%), while one episode of scaffold thrombosis (2.5%) was reported 13 days later but after DAT discontinuation. However, the clinical events reported in the BVS group were similar compared to a historical population of stent-treated patients. Moreover, OCT performed in a subgroup of 21 patients (51.2%) showed a very low incidence of BVS strut malapposition (1.1%), none of which was considered significant. Even if these results are of interest, this study had some bias for patient selection given the relatively high number of exclusion criteria, especially lesion length >24 mm, which favoured single BVS implantation14.
The other study by Diletti reported single-centre data of BVS implantation in 49 STEMI patients. Compared to the Prague 19 registry, this study had fewer exclusion criteria. At 30-day follow-up, the rate of the device-oriented endpoint was 0%, while one (2.6%) non-target-vessel MI was reported. OCT analysis, performed in a subgroup of 31 patients (63.2%), showed that seven BVS (22.7%) had more than 5% of the struts malapposed, but this finding was not associated with clinical events at early follow-up13.
What does our study add to the topic? First, this is the largest multicentre study with the longest follow-up available (six months) following BVS implantation during PPCI. Moreover, despite the absence of Killip class III-IV patients, our study was intended to expand the evaluation of BVS in the STEMI setting. In particular, BVS implantation during PPCI was allowed also in case of long lesions, and multiple BVS were implanted in 18 patients per intention-to-treat. Of interest, most of the overlapping BVS were implanted in a long segment involving a diffusely diseased distal LAD. This strategy could be of particular interest in young patients and does not preclude an eventual future surgical revascularisation once BVS resorption is complete, restoring the underlying native state of the vasculature.
To date, very few vascular data are available after BVS overlapping27, and little is known about the outcome following BVS overlapping in humans28, particularly in the highly thrombogenic STEMI setting. Our results demonstrate that the implantation of overlapping BVS during PPCI is feasible and associated with clinical results comparable to single BVS implantation. In this context, the “marker-to-marker” strategy, which was realised in 94.5% of the cases, positioning the distal marker of the proximal BVS close to the proximal marker of the distal BVS in order to limit the overlap to a maximum of 1 mm, appeared clinically effective.
Although the clinical outcomes shown in our study are similar to the ones previously reported after BVS implantation in similarly selected STEMI populations14,26, the incidence of subacute BVS thrombosis reported (independent from overlapping) may raise a note of caution over the use of this device in thrombotic lesions, particularly without a systematic intravascular imaging guide.
This multicentre registry has some limitations, mostly deriving from the observational nature of the study and the lack of a direct comparison with current standard treatment for STEMI patients. The relatively small number of patients, the limited follow-up period and the possible selection bias due to the fact that the implantation of either DES, BMS or BVS was left to the operator’s discretion preclude reaching definitive conclusions in terms of clinical outcome. Furthermore, the limited percentage (2.7%) of intracoronary imaging guidance may have influenced the clinical results. However, this study reflects contemporary, everyday interventional experience of different Italian hospitals treating STEMI patients with PPCI.
Conclusions
Single and multiple overlapping BVS implantation during PPCI is feasible and associated with encouraging immediate and midterm follow-up results. However, this initial experience does not actually support an unrestricted BVS use in STEMI patients. Larger randomised trials of head-to-head comparison versus contemporary standard of care and longer follow-up are required to assess fully the potential clinical benefit of BVS in STEMI patients.
| Impact on daily practice The BVS has been designed to accomplish the same goals as permanent DES and then to overcome their limitations by restoring native pristine vessel state. BVS have been shown to be effective for the treatment of simple and complex coronary lesions of stable patients, while few data are available on their performance in the STEMI setting. Our registry shows that BVS implantation in selected STEMI patients is feasible and associated with favourable immediate and midterm clinical outcomes, even in case of long lesions requiring multiple overlapping BVS. However, the incidence of subacute BVS thrombosis reported (independent from overlapping) may suggest the need for more studies on the matter, and might suggest a procedure guided by intravascular imaging (pre- and post-BVS implantation). Randomised trials with long-term follow-up are required to assess the potential benefit of BVS versus the actual standard of care in STEMI patients. |
Conflict of interest statement
The authors have no conflicts of interest to declare.

