
Abstract
Aims: Early clinical results after implantation of bioresorbable vascular scaffolds (BVS) in ST-elevation myocardial infarction (STEMI) are encouraging, but long-term data are missing. This study evaluates long-term outcome in STEMI patients with implanted BVS.
Methods and results: The PRAGUE-19 study is an academic study enrolling consecutive STEMI patients with the intention to implant BVS. A total of 580 STEMI patients were screened between December 2012 and March 2015; 117 patients fulfilled entry criteria and BVS was successfully implanted in 114 (97%) of them. The primary combined clinical endpoint (death, reinfarction or target vessel revascularisation) occurred in 11.5% during the mean follow-up period of 730±275 days with overall mortality of 4.4%. Definite scaffold thrombosis occurred in two patients in the early phase after BVS implantation; there was no late thrombosis. Quantitative coronary angiography (10 patients) at three years demonstrated late lumen loss of 0.2±0.33 mm and optical coherence tomography showed minimal lumen area of 5.3±1.37 mm2 and neointimal hyperplasia area of 2.9±0.48 mm2. BVS struts were still visible at three years and 99.4% of them were well apposed and covered.
Conclusions: Encouraging clinical and imaging results after BVS implantation in STEMI patients persist during long-term follow-up.
Abbreviations
BVS: bioresorbable vascular scaffold
MLD: minimal lumen diameter
OCT: optical coherence tomography
pPCI: primary percutaneous coronary intervention
QCA: quantitative coronary angiography
RVD: reference vessel diameter
STEMI: ST-elevation myocardial infarction
TVR: target vessel revascularisation
Introduction
Since they became commercially available, bioresorbable vascular scaffolds (BVS) (Abbott Vascular, Santa Clara, CA, USA) have been widely used due to the potential advantages of expected complete bioresorption within two to four years1. Theoretically, the temporary vascular scaffolding promises to decrease the risk of scaffold-related adverse events in the long term.
Several studies have shown early and midterm feasibility and safety of everolimus-eluting BVS implantation in different clinical settings, including patients with ST-elevation myocardial infarction (STEMI)2-6. The potential advantages of implanting BVS in STEMI may be related to the fact that STEMI patients are frequently younger and have less extensive coronary artery disease. On the other hand, BVS implantation during primary percutaneous coronary intervention (pPCI) may be challenging due to vasoconstriction and high thrombotic burden with possible procedural aspects (e.g., BVS undersizing, strut malapposition) having the potential to affect patient long-term outcome7.
Regarding long-term clinical follow-up after BVS implantation, favourable clinical outcome has been reported in stable patients with the longest follow-up of five years8-10. In STEMI patients, data concerning long-term follow-up, including the rate of very late BVS thrombosis, are missing. Thus, we sought to evaluate the long-term clinical follow-up of patients enrolled in the PRAGUE-19 study, including the three-year angiographic and optical coherence tomography (OCT) imaging substudy.
Methods
STUDY POPULATION
The PRAGUE-19 study is a prospective two-centre open-label study. The design and short-term results of the pilot study phase have been published previously4. Seven hundred and fifty-five consecutive STEMI patients entered the study between December 2012 and December 2015 (end of enrolment). This article represents the interim analysis of long-term clinical follow-up of patients enrolled from December 2012 until March 2015, with the possibility of evaluating at least one-year clinical outcome in this cohort. During this screening period of 27 months, 580 patients entered the study, with 117 patients fulfilling the inclusion criteria for BVS implantation. BVS was successfully implanted in 114 (97%) patients. In three patients, BVS could not be delivered to the culprit lesion and a metallic stent was used instead. The exclusion criteria were both clinical (Killip class III-IV, life expectancy less than three years, indication for oral anticoagulation, contraindication or high likelihood of non-compliance to dual antiplatelet therapy) and angiographic (infarct artery diameter <2.3 mm or >3.7 mm, lesion length >24 mm, extensive infarct artery calcifications or severe tortuosity, STEMI because of stent thrombosis or in-stent restenosis). The incidence of exclusion criteria as well as the implantation technique have been described previously11. The study protocol mandated dual antiplatelet therapy for 12 months after BVS implantation. The study was approved by the local ethics committee at each centre as well as by the national multicentric ethics committee, and written informed consent was obtained from all study patients. This study was conducted according to the Declaration of Helsinki.
CLINICAL FOLLOW-UP
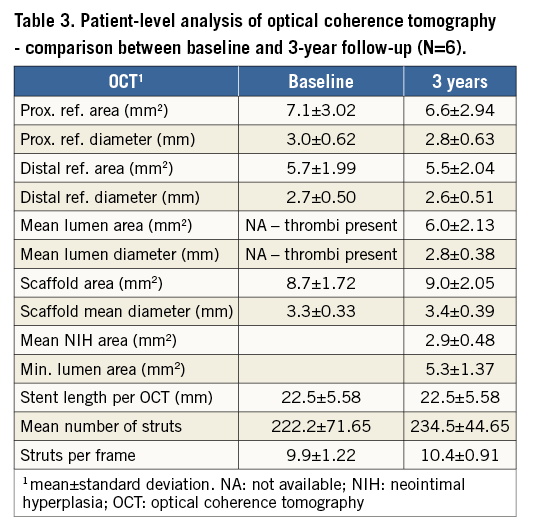
The one-year clinical follow-up was evaluated in 113 of 114 patients with implanted BVS (one patient was lost to follow-up). The two-year clinical outcomes were analysed in two pre-specified groups: (i) BVS group (69 patients available for two-year follow-up), and (ii) control group (consecutive Killip I–II class patients with a drug-eluting or bare metal stent implanted; n=127). BVS and control groups did not differ in age (60±10 vs. 63±13 years; p=0.072) or gender (male 72.5% vs. 78%; p=0.388), but there were fewer diabetic patients (10.5% vs. 26.7%; p<0.001) and right coronary artery infarctions (32.5% vs. 53.5%; p<0.001) in the BVS group. Also, the mean stent/scaffold size was larger by 0.3 mm (p<0.01) in the control group. One patient in the BVS group and five patients in the control group were lost during the two-year follow-up. The three-year clinical follow-up was evaluated in 19 patients: 10 of them had already undergone the repeat coronary angiography with OCT imaging as prescribed by the study protocol (Figure 1). The primary endpoint of the study was a combination of death, myocardial infarction and target vessel revascularisation (TVR). BVS thrombosis was defined according to the Academic Research Consortium definitions12.
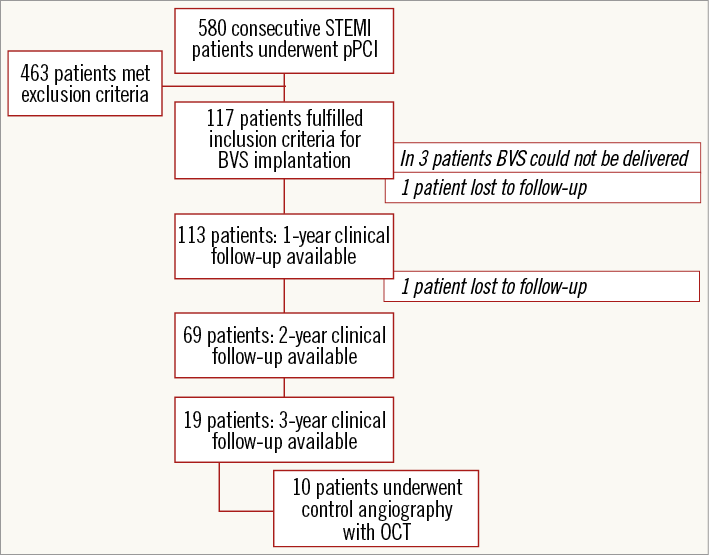
Figure 1. Scheme of patient enrolment and follow-up. BVS: bioresorbable vascular scaffold; OCT: optical coherence tomography; pPCI: primary percutaneous coronary intervention; STEMI: ST-elevation myocardial infarction
INVASIVE ASSESSMENT AT THREE-YEAR FOLLOW-UP
All patients had coronary angiography at the end of pPCI with BVS implantation, and quantitative coronary angiography (QCA) was analysed off-line. Optical coherence tomography in the acute phase was recommended but not mandatory (the most common reason not to perform OCT was patient haemodynamic instability or clinically significant arrhythmia). In a prospective per-protocol design approved by the ethics committee, invasive assessment has been repeated at three-year follow-up in 10 patients so far (Figure 1). All patients studied at the three-year time point had had OCT performed. During the procedure, intracoronary nitroglycerine was administered to all studied arteries. There were no complications and all patients were discharged home after overnight observation.
QCA ANALYSIS
The treated segments (defined as 5 mm proximal and distal to the scaffold edge) were analysed using Philips Xcelera software (Philips Medical Systems, Eindhoven, The Netherlands). Image calibration was performed with a contrast-filled 6 Fr guiding catheter. The following QCA parameters were measured after the BVS implantation: proximal and distal reference vessel diameter (RVD) defined as the largest lumen within 5 mm of the proximal and distal edges, minimal lumen diameter (MLD), defined as the smallest lumen within the scaffold, and mean scaffold diameter. Diameter stenosis was calculated from the MLD and the average of the proximal and distal RVD expressed as a percentage. Late lumen loss was calculated as MLD assessed at follow-up angiography minus MLD at baseline. Binary restenosis was defined as diameter stenosis >50% at follow-up QCA.
OCT ACQUISITION AND ANALYSIS
OCT of the infarcted vessel with implanted BVS was performed using the frequency domain C7 system with a Dragonfly™ or Dragonfly™ Duo catheter (St. Jude Medical, St. Paul, MN, USA), a pullback speed of 20 mm/sec, and an image acquisition of 100 or 200 frames/sec. OCT measurements were performed using the proprietary software for off-line analysis (ILUMIEN™ OPTIS™ system; LightLab Imaging, Westford, MA, USA) which has validated reproducibility13. OCT was analysed on a per-patient and per-frame basis. All OCT measurements were based on published standards14. Briefly, reference vessel area and diameter were measured at the site with the largest lumen within 5 mm of the proximal and distal scaffold edges. Lumen area/diameter and scaffold area/diameter were measured at vessel cross-sections at 1 mm intervals. Mean lumen/scaffold area and diameter were defined as the mean of all cross-sections within the device. Minimal lumen area was measured at the site of the smallest lumen area in the device. Neointimal hyperplasia area was computed simply by subtracting lumen area from the scaffold area – it was not possible to identify the contours reliably (and subtract the area) of the struts after three years. In the per-frame analysis, we counted all visible struts and every strut was marked optimal if it was apposed and covered by tissue. Other possibilities were: strut fracture (discontinuity), strut over side branch, malapposed strut and uncovered strut. The presence of thrombus was also recorded.
STATISTICAL ANALYSIS
Standard descriptive statistics were applied in the analysis: absolute and relative frequencies for categorical variables. Continuous variables were described using the mean supplemented with standard deviation. The statistical significance of differences between groups of patients was computed using Fisher’s exact test for two category variables, and the maximum likelihood x2 test for category variables with more than two categories. The outcome of patients was described by means of Kaplan-Meier methodology, and the statistical significance of differences in the combined clinical endpoint between groups of patients was tested using the log-rank test. The statistical significance of changes in continuous variables (QCA and OCT) between baseline and three-year follow-up was tested using a paired t-test. P-values <0.05 were considered statistically significant. Statistical analysis was computed using SPSS 23.0.0.0 (IBM Corp., Armonk, NY, USA).
Results
CLINICAL FOLLOW-UP
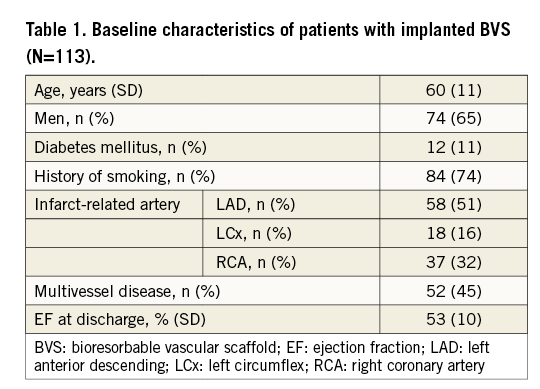
Baseline characteristics of 113 patients with implanted BVS are shown in Table 1. During the mean follow-up period of 730±275 days, the primary endpoint occurred in 13 (11.5%) patients with overall mortality of 4.4% (five deaths, four reinfarction and four TVR). Regarding the cause of death, there were two sudden deaths at 14 days and nine months after BVS implantation, there was one intracranial bleeding three days after BVS implantation, one patient died after surgical repair for post-infarction ventricular septal rupture and one patient died 11 months after BVS implantation due to renal cancer. Two reinfarctions were potentially related to BVS (early BVS thrombosis after ticagrelor discontinuation); the other two reinfarctions were not related to BVS (different coronary artery territory). As far as TVR is concerned, one event was definitely related to BVS (BVS restenosis after six months), and three others were native lesions in the same vessel not related to the BVS segment. All 13 events happened during the first year after BVS implantation. No event occurred in 69 patients between one and two years of clinical follow-up and in 19 patients between two and three years.
Two patients suffered early definite stent thrombosis (13 and 14 days after BVS implantation, both patients discontinued ticagrelor treatment). There was one case of probable stent thrombosis (sudden death at home 14 days after BVS implantation). No other definite/probable stent thrombosis occurred up to the three-year follow-up. Eighty-six percent of patients in the BVS group and 82% in the control group (p=0.683) were using monotherapy of acetylsalicylic acid at the time of two-year follow-up. The current analysis concerns patients enrolled until March 2015 but we feel it prudent to report that there was no early scaffold thrombosis in patients with BVS implanted between March 2015 and the end of study enrolment.
No differences in the primary composite endpoint during the two-year follow-up have been found between the BVS and the control group (7.2% vs. 11.8%; p=0.275). The comparison of event-free survival between both groups is shown in Figure 2.
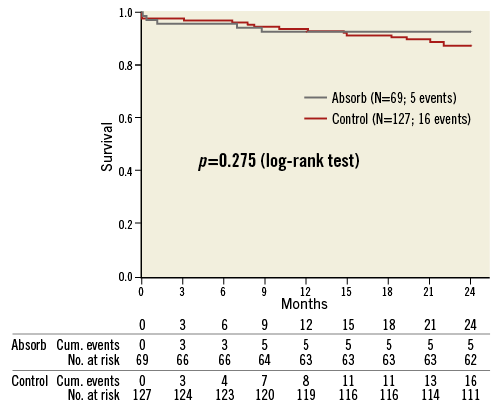
Figure 2. Kaplan-Meier event curves comparing bioresorbable vascular scaffold and control groups for an event-free survival.
INVASIVE ASSESSMENT AT THREE-YEAR FOLLOW-UP
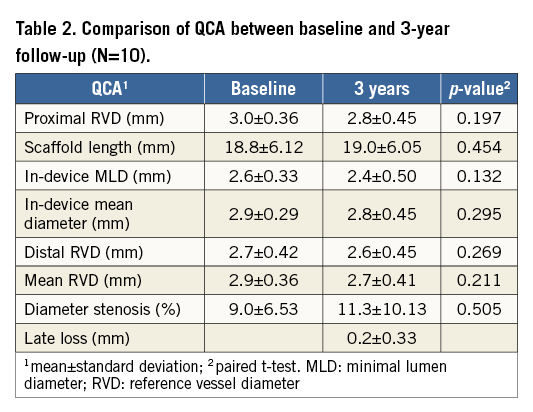
The results of QCA analysis are presented in Table 2. There are no differences in reference vessel size as well as in all other in-device parameters. Diameter stenosis was 9.0±6.53% at baseline and 11.3±10.13% (p=0.505) after three years. Late lumen loss was only 0.2±0.33 mm. There was no binary restenosis present.
Baseline OCT was available in six patients and the per-patient comparison with three-year data is presented in Table 3. Per-frame analysis was performed in all 10 patients with three-year OCT data. In total, there were 2,056 struts visible in 203 analysed frames. An optimal result was present in 2,043 (99.4%) struts. Strut discontinuity consistent with strut fracture was detected in five struts (two patients), seven struts were over the side branch, and small thrombus was present in one analysed frame. There were no malapposed or uncovered struts.
Discussion
Our study reports the first long-term clinical outcome and imaging data in STEMI patients treated with BVS during primary percutaneous coronary intervention.
There is a paucity of long-term data after BVS implantation. The only available data with follow-up longer than 12 months are from an animal model15 and the original ABSORB A and ABSORB B patient cohorts9,10. In these first-in-man studies in stable patients with mostly simple lesions, the rate of major adverse cardiac events was low - 11% at five-year follow-up of ABSORB cohort B. Regarding BVS use in STEMI, the PRAGUE-19 study is the only trial with reported results with over 12 months of follow-up16. The current study extends our recently published two-year follow-up of the pilot phase of the PRAGUE-19 study17 and presents the first three-year clinical and imaging results in this clinical setting. Interestingly, all clinical events in our study occurred in the first 12 months. It could be speculated whether this finding is related to quick BVS healing. In the TROFI II trial, stenting of culprit lesions with BVS in the setting of STEMI resulted in a nearly complete arterial healing which was comparable with that of metallic everolimus-eluting stents at six months18. The restenosis within BVS is the only event with clear, non-disputed causal relationship to BVS and was successfully treated by simple balloon dilatation. The causal relationship of all the other events was less clear – both early definite stent thromboses could be explained by ticagrelor discontinuation (non-compliance in one patient and transition from ticagrelor to clopidogrel in the other).
The bioresorbable vascular scaffold thrombosis rate became a serious concern with recent meta-analyses reporting increased risk, especially in the subacute phase19,20. Recently, several cases of very late BVS thrombosis have been published21-25. The cause of scaffold thrombosis in these reports seems to be related to: a) an implanted scaffold with large areas of strut malapposition (7-9% of struts) or strut discontinuity/fracture, or b) discontinuation of antiplatelet medication. The role of strut discontinuity is not clear, with this incidental finding detected in 40% of patients without any clinical implications26. It is reassuring to note a very low incidence of strut malapposition (0%) and discontinuity (0.2%) in our OCT data. This optimal and persistent result after BVS implantation might have prevented any thrombotic events after reducing antiplatelet medication to a single agent in the majority of our patients at 12 months.
As far as invasive assessment after three years is concerned, our results are very similar to the reported five-year angiographic follow-up of the ABSORB B cohort9. Late loss was 0.2±0.33 mm in our patients and 0.26±0.42 mm in ABSORB B five-year data. A smaller MLD of 2.02±0.45 mm in ABSORB B is probably related to smaller scaffold diameter (only 3.0×18 mm devices used) and this could also explain the higher rate of binary restenosis. Optical coherence tomography results (scaffold area, neointimal hyperplasia area) are also similar to published three-year data8. However, we did not find any scaffold area enlargement between baseline and three years. We confirm the presence of visible struts in the vessel wall (Figure 3), similar to three-year OCT data from the ABSORB B cohort8. Our data do not allow us to speculate regarding proteoglycans replacing the polylactic acid. Due to this finding we have amended the protocol: one part of our cohort will have invasive assessment delayed until five years when we can expect complete resorption9.
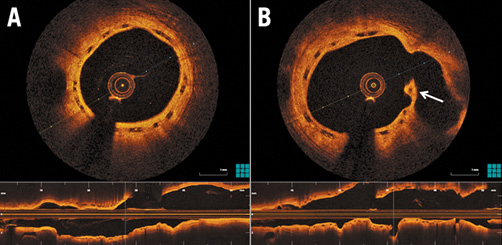
Figure 3. Three-year optical coherence tomography imaging. A) Still visible, completely covered scaffold struts with excellent apposition to vessel wall and large lumen area. B) Strut at ostium of the side branch (arrow) (confirmation of persistent, unresorbed strut).
Limitations
There are obvious limitations due to the single-arm non-randomised study design with significant selection bias due to the majority of screened patients having exclusion criteria. The moderate number of enrolled patients and very simple matching of the control group (no propensity matching possible, again due to the number of patients) do not allow us to reach any definitive conclusions, especially where infrequent events such as scaffold thrombosis are concerned. Very late scaffold thrombosis might be clinically undetected in patients with prior STEMI. Invasive assessment is certainly limited due to the small number of studied patients and should be interpreted as hypothesis-generating. The per-frame OCT analysis with over 2,000 assessed struts is more robust. Our results should be confirmed by larger randomised studies with assessment of the long-term follow-up.
Conclusions
This PRAGUE-19 study update presents the first data regarding long-term follow-up of STEMI patients treated with BVS implantation during primary percutaneous coronary intervention. Long-term clinical outcomes are encouraging. Specifically, the risk of late events associated with reducing antiplatelet medication after 12 months seems to be low. Efficacy and safety of BVS implantation in STEMI patients is supported by invasive imaging at three years.
| Impact on daily practice It is reassuring for interventional cardiologists performing often challenging pPCI that the first long-term clinical follow-up results of BVS in this setting are encouraging. It seems safe to reduce antiplatelet medication to a single agent after 12 months. Invasive imaging after three years demonstrated visible scaffold struts, good arterial healing and minimal late lumen loss. |
Funding
This study was supported by Charles University Research program P 35 and UNCE 20410.
Conflict of interest statement
P. Toušek, V. Kočka, M. Malý and P. Widimský have received occasional speakers’ honoraria from Abbott Vascular. The other authors have no conflicts of interest to declare.

