Abstract
Aims: The safety of BVS implantation in patients with a high risk for early thrombotic complications has not been studied. We report on the outcomes of patients with acute coronary syndromes (ACS) treated with bioresorbable, everolimus-eluting, vascular scaffolds (BVS).
Methods and results: 150 consecutive patients with ACS (194 lesions) treated with BVS between May 2012 and July 2013 were compared with a control group composed of 103 consecutive patients (129 lesions) who underwent everolimus drug-eluting stent (DES) implantation in the same time period. The incidence of major adverse cardiac events (MACE: death, non-fatal myocardial infarction, or reintervention) before discharge, at one month and six months was evaluated. Clinical characteristics and presentation were similar between groups. Procedural characteristics were also similar between groups, except for the use of glycoprotein IIb/IIIa inhibitors (p<0.01). Procedural success was obtained in all but two patients in the BVS group. In-hospital, 30-day and six-month MACE rates were similar between both groups (all p>0.5), with most complications occurring during the first ten days. Definite or probable in-stent/scaffold thrombosis occurred in two BVS patients and one DES patient during the index admission and it occurred in another patient in each group in the first month after BVS/DES implantation. In multivariate analysis, BVS utilisation did not influence the incidence of MACE (p>0.9).
Conclusions: BVS implantation for patients with ACS is safe, with outcomes comparable with those of drug-eluting metal stents.
Introduction
Absorb bioresorbable scaffold systems (BVS) have recently been introduced in the European market for the treatment of coronary artery stenoses. While data are now available supporting the use of these devices in the setting of type A lesions1-5 in stable coronary artery disease, evidence regarding the treatment of culprit lesions with BVS in acute coronary syndromes is not yet available.
In cases of stable plaques, multimodality imaging evidence suggests that the vascular wall behind the implanted BVS undergoes a remodelling that appears to be associated with features of plaque sealing and increased plaque stability6. These concepts are particularly attractive in patients with acute coronary syndromes, where the rupture of a plaque causes transient vascular obstruction. In this context, therapies aimed at improving plaque stability (including a combination of temporary mechanical scaffolding, antiplatelet and cholesterol-lowering therapy) might potentially be an alternative to traditional metal drug-eluting stents (DES). We report data on the clinical outcome after implantation of BVS in patients with acute coronary syndromes.
Materials and methods
All consecutive patients who received at least one BVS for the treatment of a culprit lesion in the setting of an acute coronary syndrome between May 2012 and June 2013 in our institution are included in the present report. Briefly, lesions had to be de novo, in a native coronary artery with a reference vessel diameter compatible with the use of a 2.5, 3.0 or 3.5 mm BVS (between May and September 2012 only 3.0×18 mm BVS were available). An acute coronary syndrome was defined as unstable angina (defined as acute onset, typical angina leading to admission to the emergency department and ECG changes suggestive of ischaemia), non-ST-elevation myocardial infarction (NSTEMI), or ST-elevation myocardial infarction (STEMI). Implantation of a BVS was not considered in the following cases: lesions located in the left main coronary artery, lesions involving a side branch >2 mm in diameter, calcified lesions preventing effective predilation (residual stenosis >60% of the lumen after angioplasty) as well as in-stent restenosis/thrombosis lesions. Lesions treated were defined as culprit based on angiographic evidence of intraluminal thrombus, complete vessel occlusion or stenosis >90% at quantitative coronary angiography, and ECG changes and/or regional wall abnormalities in the corresponding perfusion territory.
For the purpose of comparison, data from consecutive patients treated with XIENCE PRIME™ metal everolimus-eluting stents (Abbott Vascular, Santa Clara, CA, USA) during the same time period were also collected. The same clinical definitions and anatomical limitations were applied. Device choice was mainly based on the availability of scaffolds at the moment of the implantation, and no criteria distinguished the patients between groups. The choice of the dual antiplatelet therapy was based on current international guidelines7 and reflects the daily routine in our centre.
Procedures
The Absorb (Abbott Vascular) device consists of a polymer backbone of poly-L-lactide coated with a thin layer of a 1:1 mixture of poly-D,L-lactide polymer. The antiproliferative drug everolimus is integrated in this matrix in a dose of 100 mg of everolimus/cm2 of scaffold. Previous publications have described the structure of the device in detail8. Except for BVS utilisation, all procedures were performed according to standard techniques, and the final interventional strategy and the use of GP IIb/IIIa inhibitors post-dilatation was left to the operator’s discretion. Thrombectomy was used in all cases of complete thrombotic vessel occlusion. Weight-adjusted heparin was administered with a target activated clotting time of 250 seconds, and target lesions were treated using standard interventional techniques with mandatory predilatation. Dual antiplatelet therapy with aspirin 100 mg plus clopidogrel, prasugrel or ticagrelor was prescribed in all patients for 12 months.
Outcomes
Major adverse cardiac events (MACE) including death, non-fatal myocardial infarction (with or without ST-segment elevation), or need for revascularisation (target or non-target lesion/vessel, including planned staged revascularisations) were collected at discharge, at one month and at six-month follow-up as defined by the ARC criteria9. The definition of myocardial infarction before and after the index procedure was based on standard published criteria10. Additionally, data on device procedural performance and periprocedural safety were collected. All deaths were deemed cardiac unless proven otherwise. Patients had troponin I and creatine-phosphokinase levels measured immediately before procedure, six and 24 hours later. In-hospital and follow-up information, including records of repeat interventions (surgical and percutaneous) and re-hospitalisations were obtained through the hospital’s electronic clinical database.
Statistical analysis
Data are presented as mean±standard deviation or number (%). Continuous variables were compared with the Student’s t-test or analysis of variance, while discrete variables were compared with the Fisher’s exact tests. Cumulative survival and MACE-free survival were analysed with the Kaplan-Meier method and compared with the log-rank test. Multivariate independent predictors of 30-day outcomes were evaluated by logistic regression. All baseline and procedural characteristics were included in this analysis, and a final multivariate model was constructed by backward deletion of the least significant variables. All tests were two-tailed. MedCalc (MedCalc Software, Mariakerke, Belgium) was used for the analysis.
Results
The clinical and procedural characteristics of the 253 patients included in the present registry are presented in Table 1. As compared with those treated with metal drug-eluting stents, patients treated with BVS had a lower prevalence of hyperlipidaemia. Other than that, there were no differences between groups in clinical presentation, risk factors or lesion type.
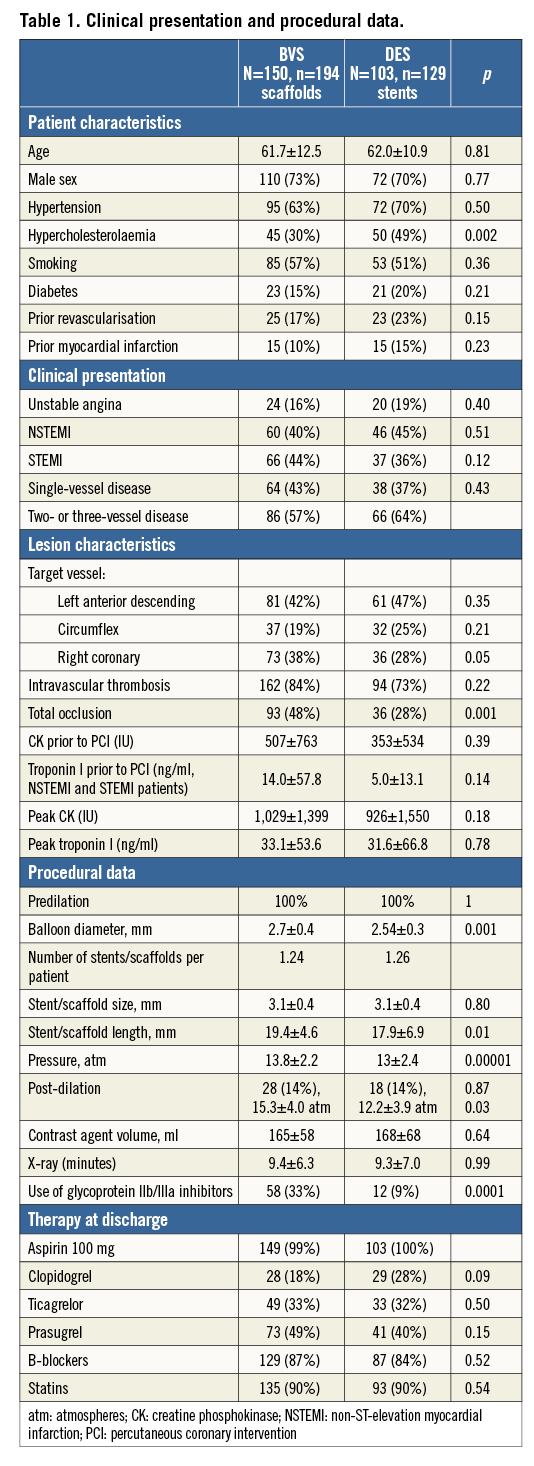
Procedural angiographic success was achieved in all attempted lesions in the metal stent group (100%) and in all but two patients in the BVS group (these two patients were treated with DES but were not included in the DES group in the present database). The balloon used for predilation was slightly larger and GP IIb/IIIa inhibitors were used more frequently in the BVS group, probably reflecting the higher incidence of thrombotic occlusion of the vessels. Of note, although the pressure used to deploy the scaffold was higher and the time of inflation was systematically longer in the BVS group (due to the different implantation technique, 2 atm pressure increase each five seconds for BVS, p<0.0001), there was no difference in the amount of contrast administered and the total x-ray time during catheterisation. Discharge therapy was the same between groups. Two representative cases are presented in Figure 1.
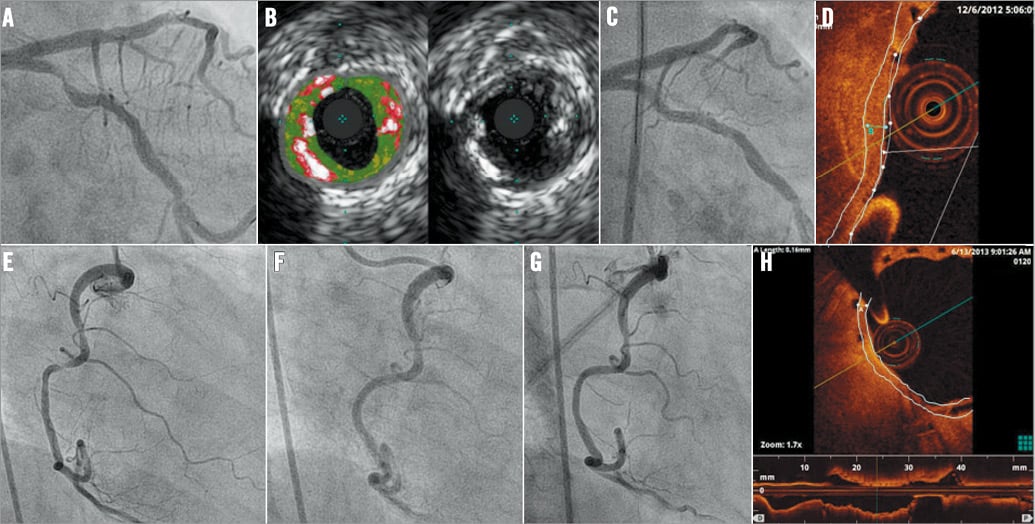
Figure 1. Representative cases. A-D) A 70-year-old woman with unstable angina and a thrombotic lesion in the proximal circumflex coronary artery (A). Intravascular ultrasound confirmed the presence of multiple layers of dense calcium and necrotic core (B). Six months after implantation of an Absorb 3.0×18 mm scaffold (C), optical coherence tomography (D) showed that the same (subintimal, calcified) lesion was covered by a ~170 µm-thick neointimal layer within and around the scaffold struts. E-H) A 40-year-old woman with NSTEMI and a thrombotic lesion in a tortuous right coronary artery with a ~90° angle (E). A 3.0×18 mm Absorb scaffold was implanted successfully: immediate result (F) and 12-month control (G). OCT (H) showed a ~170 mm-thick neointimal layer covering a large (~110° arch) subintimal fibroatheroma.
Follow-up
In-hospital and 30-day outcomes for all patients are shown in Table 2. As expected, most complications occurred in the first 10 days after the procedure (Figure 2). Two patients died during the index admission, one due to post-MI cardiogenic shock (DES group), the other (BVS group) due to post-MI ventricular septum defect with heart failure. An additional STEMI patient (not included in the database because she had received mixed BVS+DES revascularisation) died due to cardiogenic shock. Two patients had non-fatal ST-elevation MI in the BVS group, both due to acute in-BVS thrombosis (1.3%). One in-BVS thrombosis occurred immediately (15 minutes) after BVS implantation, the other three days after implantation. There was also one case of acute in-DES thrombosis (1.0%). The length of the hospital stay was similar between groups. At one month, one patient died in the BVS group (sudden death) and two patients died in the DES group (both sudden death; in one case, in-stent thrombosis was documented at autopsy). There was one documented in-scaffold (or in-stent) thrombosis in each group and three non-fatal MIs (one STEMI in the BVS and two NSTEMIs in the DES group treated with nTLR). In total, based on the ARC criteria, there were three cases of definite in-BVS thrombosis and two cases of definite in-DES thrombosis within the first 30 days after implantation. An incomplete expansion of the BVS could be evidenced at OCT in two of the three cases of definite in-BVS thrombosis (Gori T et al, in Press, J Am Coll Cardiol.). All three patients underwent emergency coronary angiography and were successfully treated with IIb/IIIa antagonists, heparin, thrombectomy and angioplasty with non-compliant balloons. During the one-month follow-up, there was one case of unexplained death (probable in-stent thrombosis according to ARC criteria) in each group. Importantly, all patients with definite/probable in-BVS thrombosis were on therapy with ticagrelor. There was also one STEMI in the BVS group and there were two NSTEMIs in the DES group; all these patients underwent urgent angiography and treatment (nTLRs). One patient in the BVS group underwent angiography for Prinzmetal’s angina. Overall, MACE incidence was 10.7% (16 events in 150 patients, six of which were planned staged nTLR) in the BVS and 15.5% in the DES group (16 events in 103 patients, nine of which were planned nTLR; p>0.8). Six-month outcome data were available for the first 77 patients in the BVS and 81 patients in the DES group. Ten patients in the BVS group underwent non-target lesion revascularisation (nTLR, in eight cases planned) within the six months after the index procedure, and there was one additional NSTEMI (nTLR) and one stroke in the DES group. In multivariate analysis, the presence of three-vessel disease (odds ratio [OR] 3.5 [95% CI: 1.4 to 8.9]; p<0.01), and NSTEMI at presentation (OR 3.7 [95% CI: 1.4 to 10.2]; p=0.01) were associated with an increased risk of MACE at 30 days. When forced into the model, BVS (p>0.9) had no impact on the occurrence of adverse events.
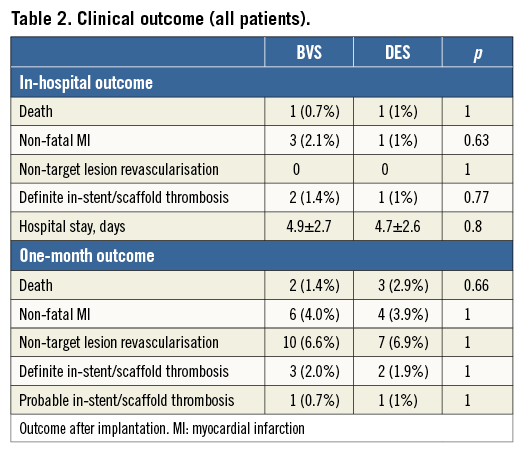
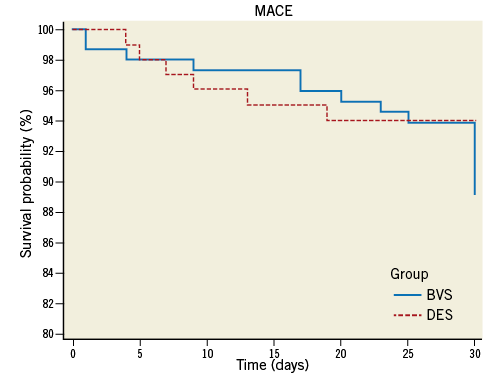
Figure 2. Cumulative MACE (major adverse cardiac events: death, non-fatal infarction or reintervention) during the first month after DES implantation (dotted line) or BVS implantation. As described in the text, most serious MACE occurred during the first 10 days in both groups, while a number of interventions were planned and occurred between 20 and 30 days after index PCI. p=0.26 (log-rank test).
Discussion
Recent studies have investigated the clinical outcome of BVS in the setting of de novo coronary artery lesions, but these analyses have mostly been limited to isolated type A lesions in patients presenting with stable angina or inducible ischaemia11-14. Theoretical advantages of BVS over traditional metal stents include the fact that the bioresorption of the scaffold allows the recovery of physiological processes of endothelial vasomotor function, vascular remodelling and, at a distance of time after implantation, lumen enlargement15. Further, spasm and thrombus burden are believed to cause late malapposition after primary PCI with metal stents. In addition, it has been hypothesised that a fully biological structure would reduce the foreign body inflammatory response associated with metal stent implantation16 and polymer exposure17. Given the role of endothelial function in determining plaque development and thrombus formation18, these considerations might be of particular importance for patients who present with an acute coronary syndrome. Finally, the concept that metal stenting definitely “seals” unstable plaques is challenged by reports of late in-stent neo-atherogenesis19.
In the setting of stable lesions, previous studies6 have reported an increase of plaque size located behind the polymeric struts of the BVS, mainly due to an increase in its fibrous and fibro-fatty content, 12 months after implantation. Also, a significant decrease in necrotic core and dense calcium tissue has been demonstrated at this time point. Importantly, the reduction of necrotic core volume was also accompanied by a reduction in the area of the necrotic core in contact with the lumen both in the scaffolded segment and in the neighbouring reference segments. Finally, the fibrous neointima on the stent surface might contribute to stabilising the plaque and limiting the luminal exposure of thrombogenic materials, as suggested in our cases presented in Figure 1. Collectively, these data suggest that the implantation of a temporary scaffold might be associated with a stabilisation of the atherosclerotic plaque, therefore providing a biological rationale for the use of BVS in the setting of ACS.
We report data from a single-centre registry investigating the safety and clinical outcomes of BVS in patients with acute coronary syndromes. Our data apparently suggest that the outcome after the implantation of these devices is comparable to that of a modern drug-eluting stent. The incidence of mortality during follow-up and that of acute or subacute stent thromboses was relatively high but comparable to that in the metal drug-eluting stent group, and not unexpected due to the use of these devices in an emergency setting and compared to previous reports20,21. The observed association with the use of ticagrelor deserves more investigation. Notably, the episodes of mortality in our series did not appear to be related to BVS use but rather to complications associated with the clinical presentation of the patients.
This study includes consecutive patients in a non-randomised design and further data are needed to provide a more definitive demonstration of safety and efficacy of this strategy. Despite this limitation, the present population is representative of “real-world” situations, and our findings may therefore be more readily applicable to daily clinical practice. In summary, implantation of BVS in patients with ACS appears to be safe, with early outcomes comparable to conventional drug-eluting metal stents. These preliminary data merit further evaluation in the context of randomised trials.
Acknowledgements
The authors are grateful to Madeleine Kress, Nadja Weiers, Selina Muxel and Zhanghua Qu for their work. This article contains parts of their doctoral theses.
Funding
This work was supported by a grant from the German Ministry of Education and Research (BMBF 01EO1003) to T. Gori, P. Wenzel and T. Münzel.
Conflict of interest statement
T. Gori, U. Hink, F. Post and T. Münzel received speaker’s honoraria from Abbott Vascular. The other authors have no conflicts of interest to declare.

