Abstract
Aims: In the drug-eluting stent (DES) era, patients with acute coronary syndrome (ACS) had a higher risk of early stent thrombosis compared with stable patients. The present study aimed to evaluate whether the same is true for the bioresorbable vascular scaffold (BVS) (Abbott Vascular, Santa Clara, CA, USA).
Methods and results: We assessed the relationship between the incidence of definite/probable scaffold thrombosis (ScT) (overall ScT and early ScT) and ACS percentage with the latest publications, including the most recent large randomised controlled trials. Out of a total study population of 13,708 devices in 45 trials, overall ScT was observed in 185 devices (1.35%) at a weighted mean follow-up period of 9.4 months, while early ScT was reported in 125 devices (0.97%) out of 12,896 devices in 44 trials. Meta-regression analysis demonstrated no significant correlation between overall/early ScT and the percentage of patients with ACS (overall ScT: R2=0.030, p=0.255; early ScT: R2=0.067, p=0.090).
Conclusions: ACS appeared to have little impact on the incidence of ScT after the implantation of BVS. Further clinical study is warranted to investigate the predictors of ScT using multivariate analysis with sufficient statistical power.
Introduction
Lipinski et al reported that studies with a higher percentage of patients undergoing percutaneous coronary intervention (PCI) for acute coronary syndrome (ACS) with bioresorbable vascular scaffolds (BVS) (Absorb; Abbott Vascular, Santa Clara, CA, USA) had a trend towards an increased risk of ScT (scaffold thrombosis) during follow-up1. This trend seems to be in line with the fact that, in the drug-eluting stent (DES) era, patients with ACS (0-3.1%) had a higher risk of early stent thrombosis compared with stable patients (0.3-0.4%)2. However, TROFI II, the most recent randomised controlled trial comparing BVS and DES in patients with ST-elevation myocardial infarction revealed a better healing score after the implantation of BVS than DES, and low event rate of scaffold thrombosis, implying the possibility of a different impact of ACS on ScT when compared to DES3.
Methods
We reassessed the relationship between the incidence of definite/probable ScT (overall ScT and early ScT [up to one month after device implantation] when available) and ACS percentage with the most recent registry data and large randomised controlled trials, including TROFI II, ABSORB Japan, ABSORB China, and ABSORB III trials in addition to those in the meta-regression analysis from Lipinski et al1,3-6. Two independent reviewers (C. Collet and T. Asano) systematically searched (August 2016) EMBASE/PubMed and available abstract data, applying the search terms “bioresorbable” AND “scaffold”. We included in the analysis only trials in which the percentage of ACS was available. The longest available follow-up was used for overall ScT when available. Definitions for ScT were consistent with Academic Research Consortium criteria7. Meta-regression analysis with weighting of studies based on the number of patients was performed to assess the correlation of ScT with the percentage of ACS of each trial. Details of the systematic review and statistical analysis are shown in the Online Appendix.
Results
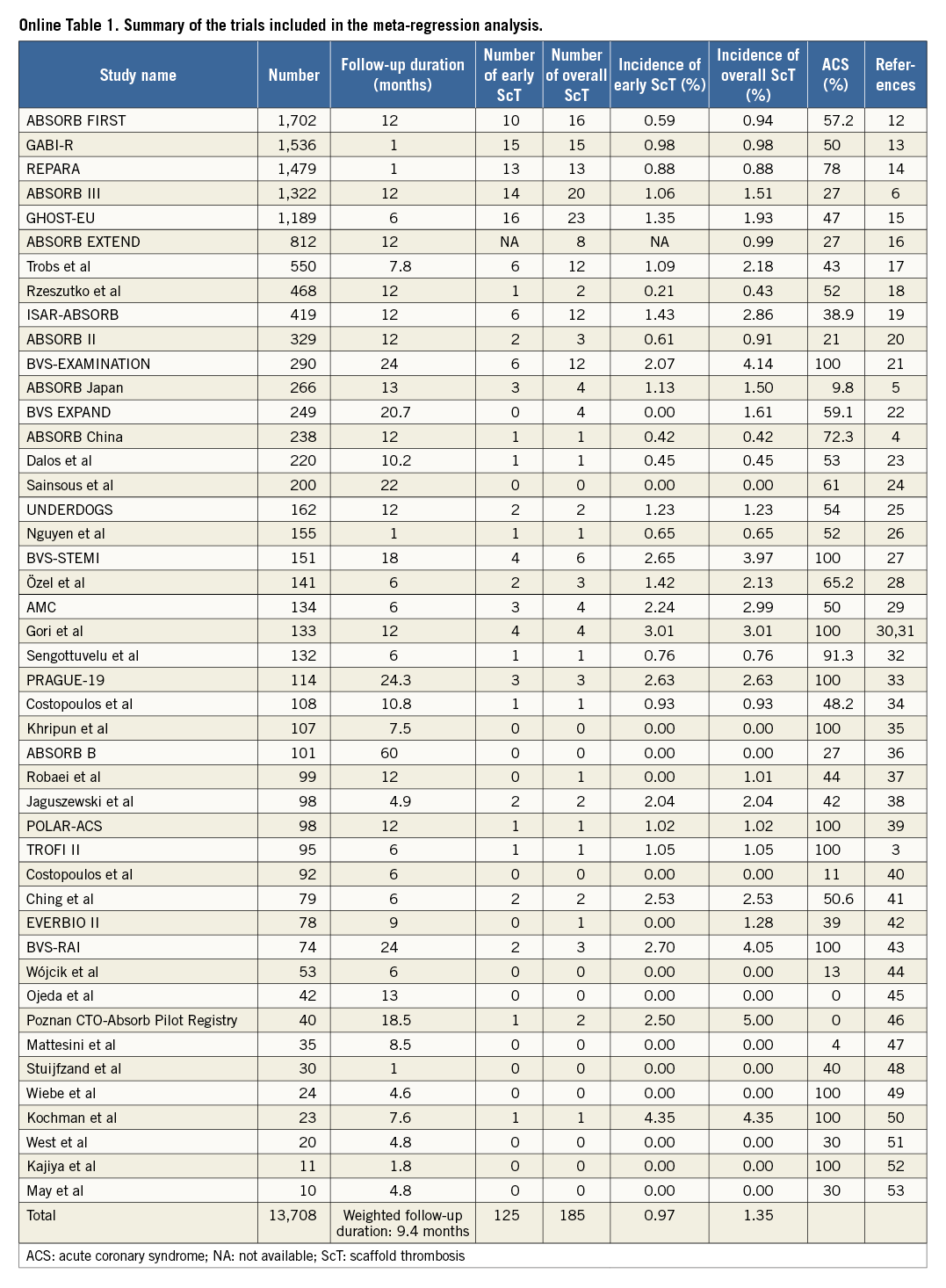
The trials included in this analysis are shown in Online Table 1. Out of a total study population of 13,708 devices in 45 trials, overall ScT was observed in 185 devices (1.35%) at a weighted mean follow-up period of 9.4 months, while early ScT was reported in 125 devices (0.97%) out of 12,896 devices in 44 trials. Meta-regression analysis demonstrated no significant correlation between overall/early ScT and the percentage of patients with ACS (overall ScT: R2=0.030, p=0.255 [Figure 1A]; early ScT: R2=0.067, p=0.090 [Figure 1B]).
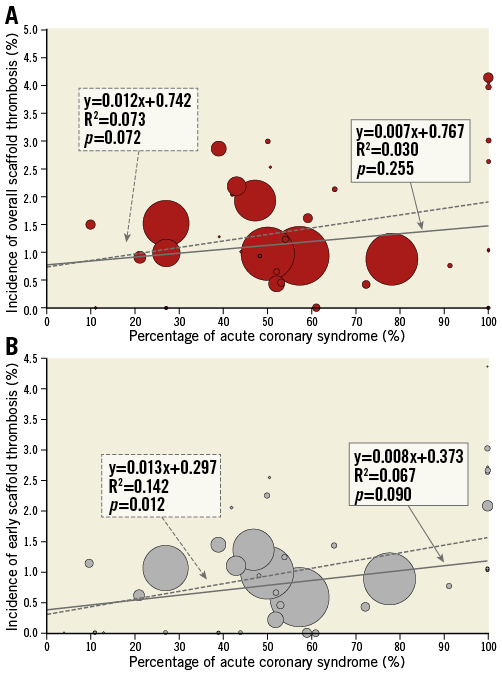
Figure 1. Correlation between definite/probable scaffold thrombosis and the percentage of patients with acute coronary syndrome. Meta-regression analysis with (solid line) and without (dotted line) weighting of studies based on the number of patients was performed to assess the correlation of overall (A) and early ScT (B) with the percentage of acute coronary syndrome of each trial.
Discussion
The present short report demonstrates that ACS appeared to have little impact on the incidence of ScT after the implantation of BVS despite our expectation based on the data in the DES era. Possible explanations are as follows. 1) BVS struts might be more embedded in the ACS lesions than in the stable lesions, resulting in less flow disturbance by less protruded struts into the lumen. The main plaque morphology in ACS lesions is fibroatheroma and thrombus in which even relatively wide struts of BVS can embed well8. 2) One of the possible causes of ScT is underdeployment of the device, which could be more frequently observed in the stable lesions with hard plaque such as fibrous or fibrocalcific plaques than in the soft tissue such as fibroatheroma and thrombus in ACS lesions. Both explanations consequently lead to the same situation. The thick protruding struts and dense distribution of the struts of underdeployed devices disrupt the laminar flow and induce endothelial shear stress disturbances. Low endothelial shear stress attenuates the endothelial expression of nitric oxide, prostacyclin I2, and tissue plasminogen activator, shifting towards a prothrombotic state9. Additionally, low endothelial shear stress may promote stent/scaffold thrombosis by inhibiting endothelial cell proliferation and retarding re-endothelialisation of the artery and strut surface10. The improvement of strut embedment and device expansion can relatively weaken the impact of ACS on the incidence of ScT when compared with DES. These speculations need to be confirmed by further precise investigations.
Of note, the present analysis should be considered hypothesis-generating only, and results should be interpreted with caution. The interpretation of results from a systematic review of observational data is particularly challenging as the included studies are often of variable quality and often do not use appropriate quality assurance processes. Selection bias due to lack of information on ACS data in some publications should also be taken into consideration. Lastly, the use of different definitions of ACS could be an important confounding factor.
Conclusions
ACS appeared to have little impact on the incidence of ScT after the implantation of BVS. To assess the impact of ACS on ScT in comparison with its impact on stent thrombosis, it is warranted to investigate the predictors of ScT using multivariate analysis with sufficient statistical power in a randomised controlled trial.
| Impact on daily practice The present systematic review and meta-regression analysis suggested that ACS has little impact on the incidence of ScT after the implantation of BVS despite our expectation based on the data from the DES era. Although the present results should be considered hypothesis-generating only, it may be conceivable that the operators who intend to use BVS do not need to avoid ACS lesions. Further clinical study is warranted to investigate the predictors of ScT with multivariable adjustment and sufficient statistical power. |
Guest Editor
This paper was guest edited by Adnan Kastrati, MD; Catheterization Laboratory, Deutsches Herzzentrum München, Technische Universität München, Munich, Germany.
Conflict of interest statement
Y. Sotomi is a consultant for GOODMAN and has received a grant from Fukuda Memorial Foundation for Medical Research and SUNRISE lab. Y. Onuma and P. Serruys are members of the Advisory Board for Abbott Vascular. The other authors have no conflicts of interest to declare. The Guest Editor has no conflicts of interest to declare.
SUPPLEMENTARY DATA
Online Appendix. Methods
META-REGRESSION ANALYSIS
We reassessed the relationship between the incidence of definite/probable ScT (overall ScT and early ScT [up to one month after device implantation] when available) and acute coronary syndrome (ACS) percentage with the most recent registry data and large randomised controlled trials, including TROFI II, ABSORB Japan, ABSORB China, and ABSORB III trials in addition to those in the meta-regression analysis from Lipinski et al1,3-6.
Two independent reviewers (C. Collet and T. Asano) systematically searched (August 2016) EMBASE/PubMed and available abstract data, applying the search terms “bioresorbable” AND “scaffold”. We also obtained presentation slides of the late breaking clinical trials (including EuroPCR, Transcatheter Cardiovascular Therapeutics and American College of Cardiology meetings). We included studies in human patients: 1) who underwent PCI for obstructive coronary artery disease; 2) with at least one-month clinical follow-up data; and 3) who underwent placement of the Absorb BVS. We excluded studies with inadequate data for abstraction, duplication of data, studies using other bioresorbable scaffolds (polymer or metallic), and studies using metallic stents with a bioresorbable polymer coating. We included case reports and case series when the denominators were available. Data were abstracted by the same two investigators (C. Collet and T. Asano) in accordance with PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines11. When available, we abstracted clinical data in studies comparing clinical outcomes of BVS and DES which predominantly used everolimus-eluting permanent metallic stents. We included in the analysis only trials in which the percentage of ACS was available. The longest available follow-up was used for overall ScT when available. Definitions for ScT were consistent with Academic Research Consortium criteria7.
STATISTICAL ANALYSIS
Meta-regression analysis with weighting of studies based on the number of patients was performed to assess the correlation of ScT with the percentage of ACS of each trial. Weight was calculated using the following formula:
Wi=[Pi(1–Pi)/Ni]–1/2
i: study identification; N: number of devices; P: proportion of ScT; W: weight.
Only in the cases with the proportion of zero was an absolute ScT number of 0.5 instead of zero employed for the calculation of the proportion in order to compute the weight properly. All statistical analyses were performed with SPSS, Version 23.0.0 (IBM Corp., Armonk, NY, USA).