
Abstract
Aims: The aim of this study was to investigate the long-term safety and efficacy of biodegradable scaffolds and metallic stents.
Methods and results: We analysed a total of 91 randomised controlled trials with a mean follow-up of 3.7 years in 105,842 patients which compared two or more coronary metallic stents or biodegradable scaffolds and reported the long-term clinical outcomes (≥2 years). Network meta-analysis showed that patients treated with the Absorb bioresorbable vascular scaffold (BVS) had a significantly higher risk of definite or probable scaffold thrombosis (ScT) compared to those treated with metallic DES. The risk of very late ScT was highest with the Absorb BVS among comparators. Pairwise conventional meta-analysis demonstrated that the elevated risk of ScT with Absorb BVS compared to cobalt-chromium everolimus-eluting stents was consistent across the time points of ≤30 days (early), 31 days – 1 year (late) and >1 year (very late) ScT. In addition, target lesion failure rates were significantly higher in the Absorb BVS cohort, driven by both increased risk of target vessel myocardial infarction and ischaemia-driven target lesion revascularisation.
Conclusions: Absorb BVS implantation was associated with increased risk of long-term and very late ScT compared to current-generation metallic DES. The risk of ScT occurred with a rising trend beyond one year. Systematic review registration: PROSPERO CRD42017055987.
Abbreviations
ARC: Academic Research Consortium
BES: Biolimus A9-eluting stent
BMS: bare metal stent
BP: biodegradable polymer
BVS: bioresorbable vascular scaffold
CI: confidence interval(s)
CoCr: cobalt-chromium
DAPT: dual antiplatelet therapy
DES: drug-eluting stent
dual DES: polymer-free sirolimus- and probucol-eluting stent
EES: everolimus-eluting stent
E-ZES: Endeavor zotarolimus-eluting stent
H-SES: Orsiro hybrid SES
MI: myocardial infarction
OR: odds ratio(s)
PES: paclitaxel-eluting stent
PtCr-EES: platinum-chromium everolimus-eluting stent
R-ZES: Resolute zotarolimus-eluting stent
ScT: scaffold thrombosis
SES: sirolimus-eluting stent
ST: stent thrombosis
TLF: target lesion failure
TLR: target lesion revascularisation
TVR: target vessel revascularisation
Introduction
Bioresorbable coronary scaffolds were developed with the promise of overcoming the long-term clinical implications following deployment of permanent coronary metallic stents1. Despite recent design iterations of contemporary drug-eluting stents (DES), permanent metallic stent deployment entails the risk of late stent failure attributed to repeat target lesion revascularisation, late and very late stent thrombosis (ST), and in-stent neoatherosclerosis2,3. The Absorb™ bioresorbable vascular scaffold (BVS; Abbott Vascular, Santa Clara, CA, USA), the only US FDA-approved biodegradable scaffold, delivers transient vascular support after revascularisation with properties of drug elution and bioresorption over a period of three to four years4. The prospect with the Absorb BVS was the comparable performance with contemporary metallic DES by three years, with no further increase in device-related adverse events thereafter5.
Previous studies have shown similar one-year clinical outcomes between the Absorb BVS and contemporary metallic DES6,7. However, meta-analyses have indicated an increased risk of ST and myocardial infarction (MI) with the Absorb BVS at one year8,9. The degradation of the scaffold occurs at one to four years post implantation, and the clinical benefits are anticipated to emerge after one year10. It has also been hypothesised that the return of vasomotor function, restoration of physiological wall shear stress distribution, and healing process after Absorb BVS implantation would lead to better long-term clinical outcomes11.
However, there is still limited evidence regarding the long-term safety of the Absorb BVS and contemporary DES. Since the recently presented AIDA trial demonstrated higher incidence of scaffold thrombosis (ScT) with Absorb BVS compared with metallic DES over two years, a number of meta-analyses have indicated the higher thrombogenicity with Absorb BVS12. In our study, we aimed to compare the long-term safety and efficacy of Absorb BVS, contemporary DES and bare metal stents (BMS) by pooling all the available randomised clinical trials with ≥2 years of follow-up. We performed multiple treatment comparison network meta-analysis as well as conventional pairwise frequentist meta-analyses.
Methods
STUDY DESIGN
This study reports meta-analyses of randomised controlled trials comparing two or more coronary stents or scaffolds in patients undergoing percutaneous coronary intervention which reported long-term clinical outcomes (≥2 years). Eligible study criteria and the electronic search strategy have been published previously13. Since the purpose of this study was to compare long-term outcomes, the trials with clinical outcomes <2 years were excluded. In this study, we reported network meta-analysis as well as conventional frequentist meta-analysis. First, hierarchical Bayesian network meta-analysis models were constructed for multiple treatment comparisons incorporating direct and indirect evidence from all eligible trials. Next, conventional frequentist meta-analysis was also carried out to provide better understanding of ST risks according to each time domain. Trials comparing Absorb BVS and cobalt-chromium everolimus-eluting stents (CoCr-EES) were pooled for each time period after index intervention. The study protocol was registered on the PROSPERO database of systematic reviews (number: CRD42017055987). This study was exempt from review by the Seoul National University Bundang Hospital Institutional Review Board.
ELIGIBILITY CRITERIA AND SEARCH STRATEGY
Twelve study stents were compared: (1) BMS; (2) paclitaxel-eluting stents (PES; Boston Scientific, Marlborough, MA, USA); (3) sirolimus-eluting stents (SES; Cordis, Cardinal Health, Milpitas, CA, USA); (4) Endeavor zotarolimus-eluting stents (E-ZES; Medtronic, Minneapolis, MN, USA); (5) CoCr-EES (Abbott Vascular and Boston Scientific); (6) platinum-chromium everolimus-eluting stents (PtCr-EES; Boston Scientific); (7) biodegradable polymer (BP)-EES (Boston Scientific); (8) Resolute™ zotarolimus-eluting stents (R-ZES; Medtronic); (9) BP Biolimus A9-eluting stents (BP-BES; Biosensors, Singapore, and Terumo, Tokyo, Japan); (10) Orsiro hybrid SES (H-SES; Biotronik, Bülach, Switzerland); (11) polymer-free sirolimus- and probucol-eluting stents (dual DES; B. Braun, Melsungen, Germany); and (12) Absorb BVS. Further details concerning study methods are described in the Supplementary Appendix.
OUTCOME MEASURES
The principal safety endpoint was the long-term risk of definite or probable ScT or ST defined according to the Academic Research Consortium (ARC)14. Thrombosis rates were classified as early (≤30 days), late (31 days – 1 year), and very late (>1 year) according to the time of onset after the index procedure. The key secondary endpoint was definite ST/ScT defined according to the ARC criteria. Secondary endpoints of network meta-analysis included all-cause death, cardiac death, and MI, target vessel revascularisation (TVR) and target lesion revascularisation (TLR). Secondary endpoints of frequentist conventional meta-analysis were target lesion failure (TLF), cardiac death, target vessel MI, and ischaemia-driven or clinically driven TLR.
DATA SYNTHESIS AND ANALYSIS
Both multiple treatment comparison network meta-analysis and frequentist pairwise meta-analysis were carried out in this study. Data were presented as relative odds ratios (OR) with 95% credible intervals (CrI) for network meta-analysis, OR with 95% confidence intervals (CI) for frequentist meta-analysis. Statistical methods are detailed in the Supplementary Appendix.
Results
STUDY SELECTION AND SYSTEMATIC REVIEW
A total of 91 trials including 105,842 patients were identified as having reported long-term clinical outcomes (≥2 years). The flow chart of the network meta-analysis is shown in Supplementary Figure 1. The network plot had a polygonal network configuration with mixed connections (Figure 1). While BMS, PES, SES, E-ZES, CoCr-EES, R-ZES, and BP-BES had fully closed loops, the others had limited sample size and comparisons. BP-EES was compared only with PtCr-EES. Seven trials tested the Absorb BVS, six with CoCr-EES, and one with PtCr-EES and BP-BES. There was little evidence of publication bias as shown in the comparison-adjusted funnel plot (Figure 2).
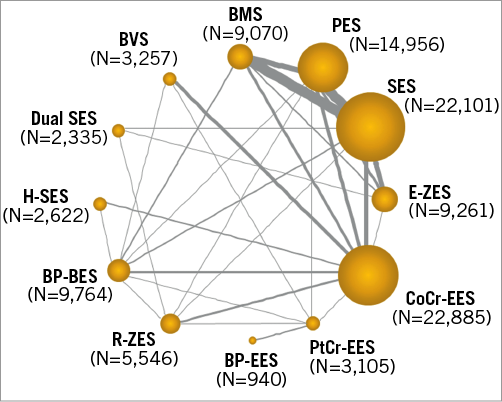
Figure 1. Network plot of eligible trials. Each stent is represented by a node. The size of the node is proportional to the sample size randomised to each stent, whereas the thickness of the line connecting the nodes is proportional to the total randomised sample size in each pairwise treatment comparison.
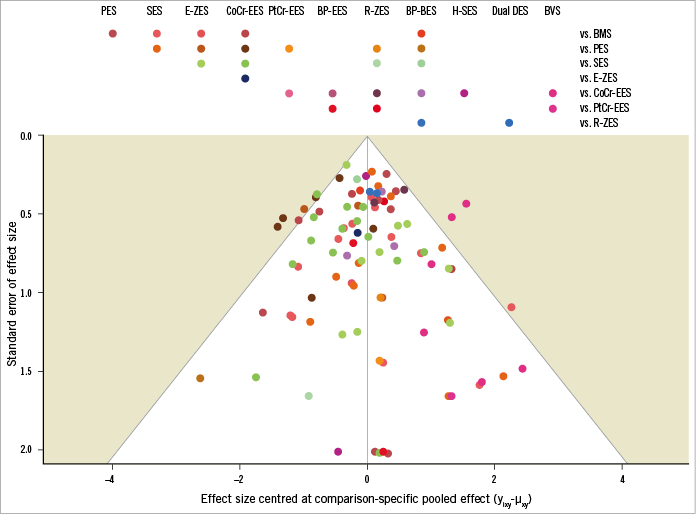
Figure 2. Comparison-adjusted funnel plot. Each dot represents a single study. The x-axis shows the centred odds ratios, while the y-axis shows the standard error of the effect estimate.
Characteristics of the included trials are summarised in Supplementary Table 1. Follow-up duration ranged from two to ten years with a weighted mean of 3.7 years. There were seven trials with a three-arm design and one trial with a four-arm design. Many of the BVS trials had stringent inclusion and exclusion criteria. The ABSORB II (N=501), ABSORB III (N=2,008), ABSORB Japan (N=398), and ABSORB China (N=475) trials enrolled stable or unstable angina patients, and excluded clinically or angiographically high-risk patients. ABSORB-STEMI TROFI II (N=192) exclusively enrolled patients with ST-segment elevation MI, and the EVERBIO II (N=238) and AIDA (N=1,845) trials had an “all-comers” design.
DEVICE THROMBOSIS
A total of 84 trials with a grand total of 99,112 patients contributed to the primary endpoint analysis, definite or probable ST/ScT at two years or longer. The ORs and CrIs for each pair of comparisons derived from the Bayesian random effects model are shown in Table 1. The Absorb BVS had a significantly higher risk of long-term ScT compared to R-ZES, E-ZES, BP-BES, dual DES, CoCr-EES, H-SES, and BP-EES. In addition, ST risk for PES, BMS, and SES was significantly higher than for R-ZES (borderline significance compared with SES), E-ZES, BP-BES, CoCr-EES, and H-SES. Forest plots visually indicate the relative risks of BVS, PES, BMS, and SES compared to other devices (Figure 3). The probability of the rank of each device was (BP-EES ≥ H-SES ≥ CoCr-EES ≥ dual DES ≥ PtCr-EES ≥ BP-BES ≥ E-ZES≥ R-ZES) > (SES ≥ BMS ≥ PES) > BVS, as shown in Figure 4. Network meta-regression analyses showed none of the study baseline characteristics significantly altering the relative treatment effects between the study stents (Supplementary Table 2). The risk of very late ScT (>1-year) of BVS was significantly higher than any other comparators except PtCr-EES and BP-EES (Supplementary Table 3). BMS showed a lower very late ST risk than SES and PES as well as BVS.
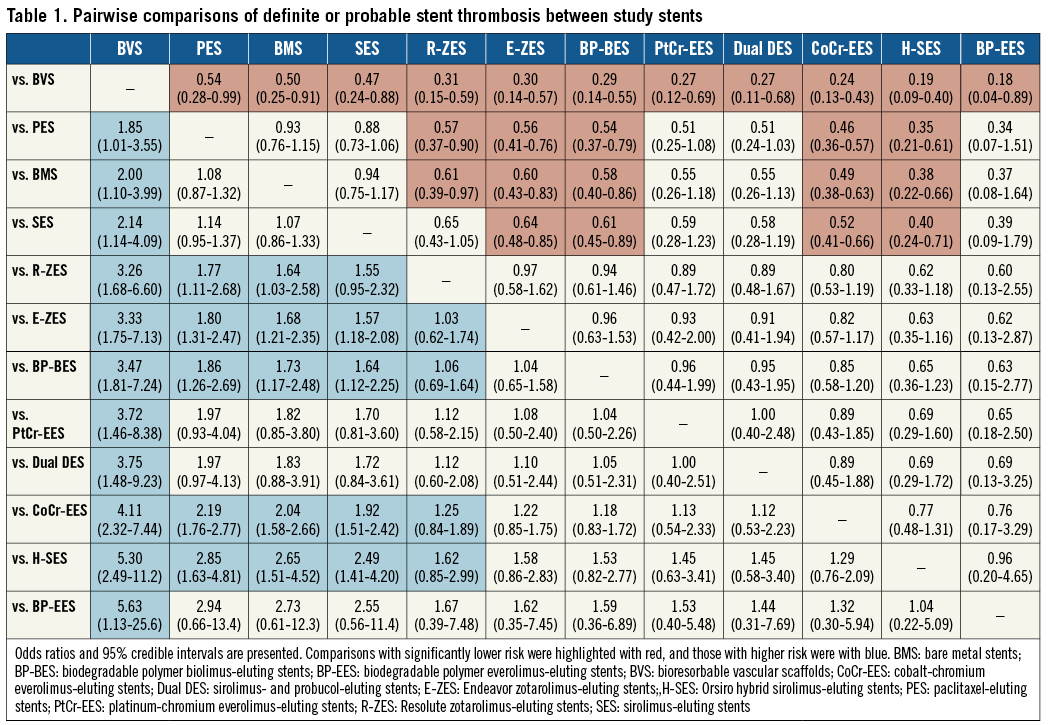
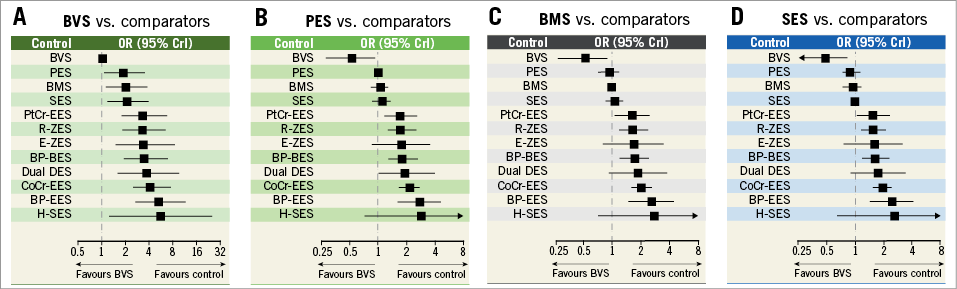
Figure 3. Network meta-analysis for long-term definite or probable stent thrombosis. A) BVS vs. comparators. B) PES vs. comparators. C) BMS vs. comparators. D) SES vs. comparators. The squares and horizontal lines indicate pairwise odds ratios (OR) and their 95% credible intervals (CrI) for definite or probable stent thrombosis.
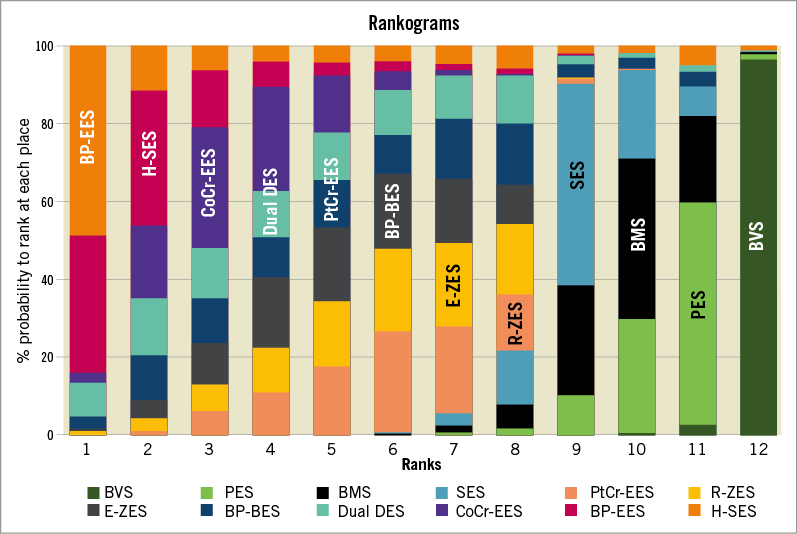
Figure 4. Ranks for study devices regarding definite or probable stent thrombosis. Bar plots for the ranking probabilities of competing stents. The possible rank of each treatment (from best to worst according to the outcome) is on the horizontal axis. The size of each bar corresponds to the probability of each treatment to be at a specific rank.
Pairwise meta-analysis was carried out pooling trials directly comparing BVS and CoCr-EES (Figure 5). Six trials with 5,418 patients contributed to the analysis. The pooled risk of long-term ScT of BVS was significantly higher than that of CoCr-EES. When ScT rates were split into early, late, and very late, the risk of very late ScT was significantly higher at all time points. There was a numerical trend towards a higher risk estimate with a more delayed time frame.
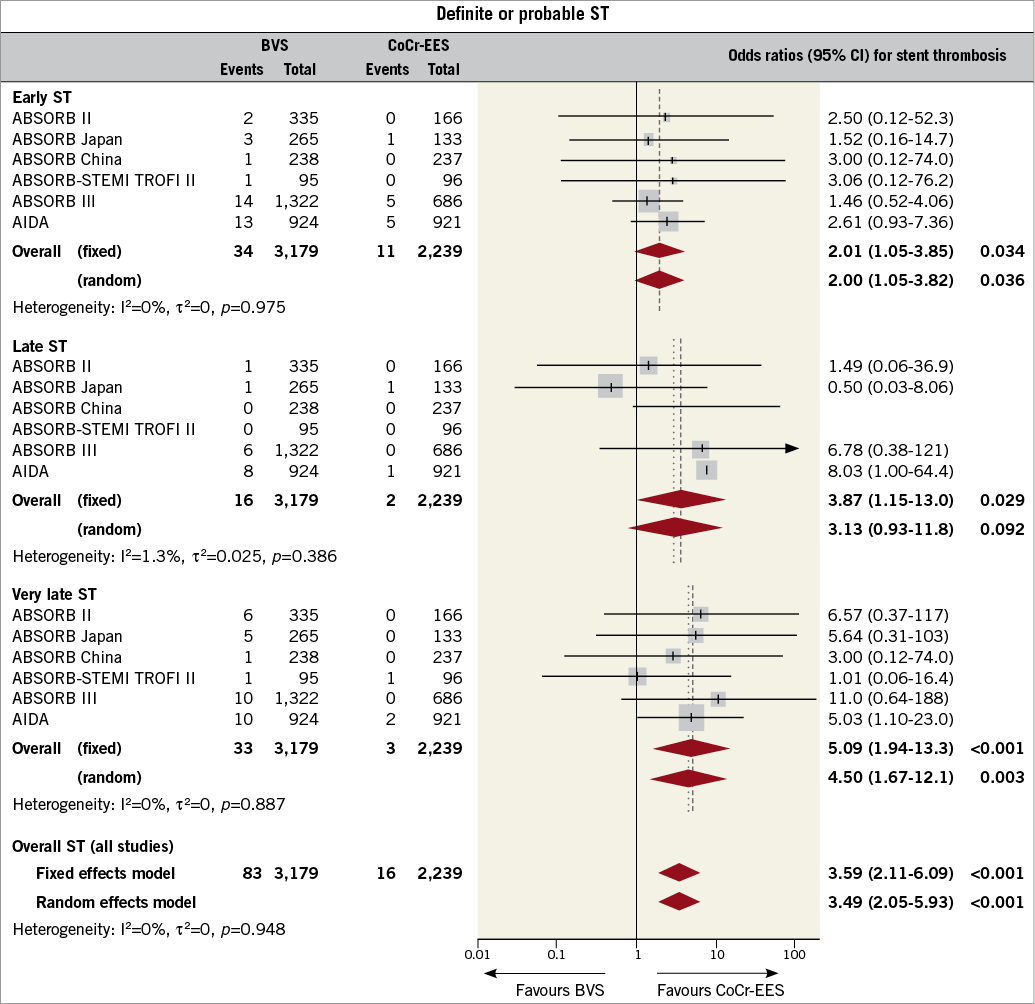
Figure 5. Frequentist meta-analysis for definite or probable stent thrombosis (ST) comparing bioresorbable vascular scaffolds (BVS) and cobalt-chromium everolimus-eluting stents (CoCr-EES). Forest plot with odds ratios (OR) for long-term definite or probable ST with BVS compared to CoCr-EES for individual trials and the pooled population. The squares and the horizontal lines indicate the ORs and the 95% confidence intervals (CI) for each included trial. The size of each square is proportional to the statistical weight of a trial in the meta-analysis.
Network meta-analysis for definite ST showed results similar to definite or probable ST (Supplementary Table 4). The probable rank of each device was as follows: (CoCr-EES ≥ H-SES ≥ E-ZES ≥ BP-EES ≥ PtCr-EES ≥ BP-BES ≥ R-ZES ≥ dual DES) > (BMS ≥ SES ≥ PES ≥ BVS). In terms of very late definite ScT, BVS showed a significantly higher risk than most of the DES (Supplementary Table 5). Frequentist meta-analysis for definite ScT/ST also showed that the pooled risk of long-term definite ScT was significantly higher with BVS than with CoCr-EES (Supplementary Figure 2).
Secondary endpoints
Network meta-analyses for all-cause death, cardiac death, MI, TVR, and TLR are shown in the electronic supplement (Supplementary Table 6-Supplementary Table 10). Network meta-analysis showed that BVS was associated with an increased risk of MI compared to SES, BP-BES, CoCr-EES, R-ZES, E-ZES, dual DES, PtCr-EES, and H-SES (Supplementary Table 8). BVS showed similar performance as compared with other DES, and significantly better than BMS in terms of TVR and TLR (Supplementary Table 9, Supplementary Table 10).
Conventional frequentist meta-analyses were carried out for TLF and its components. TLF did not differ at one year, but was significantly higher with BVS than CoCr-EES when the follow-up was extended to the long term (Supplementary Figure 3). The risk of cardiac death was similar both at one year and at the extended follow-up (Supplementary Figure 4). The increase in TLF was driven by both target vessel MI and ischaemia-driven TLR. The pooled risk of target vessel MI was significantly higher with BVS at any time period (Supplementary Figure 5). While TLR did not differ significantly at one year, it diverged and showed a statistically significant difference when the follow-up was extended (Supplementary Figure 6).
Discussion
Our study is among the first network meta-analyses to report long-term clinical outcomes following deployment of biodegradable scaffolds15. Our observations indicate that the risk of device thrombosis after Absorb BVS deployment continues to rise beyond one year. BVS deployment was associated with a higher risk of late or very late ScT compared to contemporary second-generation DES. In addition, the Absorb BVS showed elevated risks of TLF, MI and ischaemia-driven TLR.
Based on our prior observations, with this new evidence we deliver novel insights related to the long-term safety and efficacy following biodegradable scaffold deployment: the rising OR for Absorb BVS vs. CoCr-EES from 2.28 (95% CrI: 1.07-6.29) at one year, to 3.86 (95% CrI: 2.38-6.98) after a mean follow-up of 3.7 years13. The increment originated from a continuous increase of ScT risk beyond one year. Stent design iterations and material properties optimisation adopted in current-generation DES have minimised the risk of very late ST to 0.5%/year or lower especially with second-generation DES13,16. The concept of vascular restoration with the Absorb BVS was anticipated to lead to long-term safety after complete biodegradation of the scaffold. However, recent evidence indicates the dramatically increased risk of very late ScT with Absorb BVS even at two to three years17,18.
Cumulative evidence suggests that the higher thrombogenicity observed with biodegradable scaffolds occurs due to suboptimal deployment techniques in combination with the bulky scaffold’s strut design. The larger strut thickness of Absorb BVS in the range of 157 μg provides a larger platform profile in both the crimped and the expanded stage that induces local haemodynamic alterations prone to platelet activation19. An optical coherence tomography study with simulation modelling showed low endothelial shear stress zones created between the strut surfaces of the Absorb BVS20, which may predispose to acute thrombogenicity21. An animal ex vivo model also demonstrated increased acute thrombogenicity of the Absorb BVS22.
The physical property of the polymer-based Absorb BVS which enables greater acute recoil and strut fracture compared to contemporary metallic stents needs to be considered as well. Prior studies have indicated that incomplete lesion coverage, underexpansion, and strut malapposition contribute to Absorb BVS thrombosis, similar to metallic stent thrombosis23. More recently, it was suggested that late strut discontinuity may cause vulnerability to scaffold thrombotic events24. In this context, the critical role of judicious patient and lesion selection is imperative. Also, a specific implantation technique for Absorb BVS deployment is strongly encouraged.
Another potential explanation for late or very late ST risks is vascular inflammation. In a study by Virmani et al using a porcine coronary model, mild to moderate inflammatory responses were observed with the Absorb BVS25. While inflammation was absent with CoCr-EES, low-grade inflammation persisted throughout 42 months. Polymer-induced inflammation has previously been associated with ST events, especially late and very late ST26. However, it needs to be mentioned that poly-L lactide and poly-D, L lactide, which are the main materials used in the current Absorb BVS, have also been used in biodegradable polymer metallic DES as well as in other medical devices. What is the most prevailing factor associated with inflammation and whether low-grade inflammation is associated with late thrombogenicity needs to be assessed in further studies.
Evidence following observations from the ABSORB II and III randomised clinical trials strongly encourages a judicious patient and lesion selection and optimisation. Puricel et al also demonstrated a significant reduction in the incidence of scaffold thrombosis after employing a BVS-specific implantation strategy27. Stone et al pooled the results of the prospective ABSORB II, III, China, Japan, and EXTEND trials, also showing the importance of vessel sizing and operator technique28. The choice and duration of dual antiplatelet therapy (DAPT) is currently debated. While the current guidelines recommend DAPT for at least six to 12 months after implantation of contemporary DES, it is still unclear how long DAPT should be maintained for the patients who were treated with biodegradable scaffolds29.
Although the manufacturer halted commercial production of the first-generation Absorb BVS, a second-generation BVS with a thinner strut profile of 99 μm and expansion limit of >0.75 mm over nominal diameter has been tested experimentally and will undergo first-in-man studies soon. Other manufacturers are also developing polymer-based scaffolds with thinner strut profiles such as (1) DESolve novolimus-eluting scaffold (Elixir Medical Corporation, Sunnyvale, CA, USA) with a strut thickness of 120 μm, (2) MeRes100™ sirolimus-eluting hybrid cell design scaffold with a strut thickness of 100 μm (Meril Life Sciences, Vapi, India), and (3) Fantom® scaffold with a strut thickness of 125 μm (REVA Medical, San Diego, CA, USA). Obviously, these new-generation scaffolds will need to be tested against the best metallic stents to get to the point where evaluation of the long-term promise of biodegradable scaffolds can be made30.
There were additional important findings in this study. First, newer-generation DES showed an excellent long-term safety profile. Most contemporary DES had a lower risk of long-term ST than BMS and first-generation DES. Second, BMS were superior to first-generation DES in terms of very late ST. In our previous work, BMS were shown to be associated with the highest risk of early ST9. However, this study showed that BMS had safer profiles in terms of late and very late ST, comparable to those for CoCr-EES. The finding is consistent with the old belief that a bare metal surface is superior in terms of long-term safety. Third, there were no significant differences between biodegradable polymer and durable polymer metallic DES. In our previous network meta-analysis, BP-BES were shown to have significantly higher risk of ST at one year than CoCr-EES and H-SES31. However, this study showed that the longer-term ST risk of BP-BES may be considered comparable to them.
Limitations
This study has several limitations. First, the sample sizes of new devices including Absorb BVS were small, and follow-up duration was limited. Ongoing studies with larger sample size and longer-term follow-up may further elucidate the safety, efficacy and overall performance of the Absorb BVS. Secondly, the connection of the BVS arm in the evidence network was only partial. Most of the evidence came from the direct comparison between the Absorb BVS and CoCr-EES, while indirect evidence was weak. Third, the trials analysed in this study had a variety of patient characteristics, follow-up, and medication protocols.
Conclusions
The Absorb BVS was associated with an increased risk of very late ScT compared to current-generation metallic DES after a mean follow-up of 3.7 years. While previous studies have shown the Absorb BVS to be associated with an increased risk of late ScT compared to contemporary DES, this study suggests that the risk is not attenuated and rather continues to widen beyond one year.
| Impact on daily practice Device thrombosis risk after Absorb BVS implantation continues to increase beyond one year. The Absorb BVS is associated with an increased risk of long-term and very late device thrombosis as well as target lesion failure compared to current-generation metallic DES. It is anticipated that newer iterations of BVS will overcome the current issue of scaffold thrombosis. |
Conflict of interest statement
The authors have no conflicts of interest to declare.
References 32 to 279 can be found in the online version of this paper.
Supplementary data
Supplementary Appendix. Study methods.
Supplementary Table 1. Study characteristics of included trials.
Supplementary Table 2. Results from network meta-regression adjusted for covariates in terms of long-term definite or probable stent thrombosis.
Supplementary Table 3. Pairwise comparisons of very late definite or probable stent thrombosis between study stents.
Supplementary Table 4. Pairwise comparisons of long-term definite stent thrombosis between study stents.
Supplementary Table 5. Pairwise comparisons of very late definite stent thrombosis between study stents.
Supplementary Table 6. Pairwise comparisons of all-cause death between study stents.
Supplementary Table 7. Pairwise comparisons of cardiac death between study stents.
Supplementary Table 8. Pairwise comparisons of myocardial infarction between study stents.
Supplementary Table 9. Pairwise comparisons of target vessel revascularisation between study stents.
Supplementary Table 10. Pairwise comparisons of target lesion revascularisation between study stents.
Supplementary Figure 1. PRISMA flow diagram of systematic review.
Supplementary Figure 2. Frequentist meta-analysis for definite stent thrombosis (ST).
Supplementary Figure 3. Frequentist meta-analysis for target lesion failure (TLF).
Supplementary Figure 4. Frequentist meta-analysis for cardiac death.
Supplementary Figure 5. Frequentist meta-analysis for target vessel myocardial infarction (TV-MI).
Supplementary Figure 6. Frequentist meta-analysis for ischaemia-driven or clinically driven target lesion revascularisation (TLR).
To read the full content of this article, please download the PDF

