
Abstract
Aims: The present multicentre prospective study, IT-DISAPPEARS, was designed with the aim of evaluating early and long-term clinical outcomes of the Absorb BVS in patients with long coronary lesions and/or multivessel coronary artery disease. The aim of this article is to present the one-year clinical results of this study.
Methods and results: Between November 2014 and January 2016, we enrolled 1,002 patients undergoing BVS implantation (long lesion [≥ 24 mm] of a single vessel in 80.4%, at least two BVS in two or three coronary vessels in 8.6% and both criteria in 11%). Clinical presentation was an acute coronary syndrome in 59.8% of patients, including ST-elevation myocardial infarction in 21.8%. The primary endpoint was the device-oriented composite endpoint (DOCE) of cardiac death, target vessel MI, and ischaemia-driven TLR at one year. We implanted 2,040 BVS according to a pre-specified technique. One-year follow-up was available in 956 patients (95.4%). The rate of DOCE was 9.9% (95 patients). Cardiac death occurred in five patients (0.5%), while target vessel MI and TLR each occurred in 45 (4.7%) patients. The one-year rates of all-cause death, non-fatal MI, and any revascularisation were 1.2%, 5.4%, and 10.9%, respectively. The rate of definite/probable scaffold thrombosis was 0.9%.
Conclusions: This is the first study specifically investigating the Absorb technology in patients with a high atherosclerotic burden and multivessel disease. The mandatory adherence to a pre-specified implantation technique led to minimising the risk of device failure reported by other studies, in particular with respect to the rate of DOCE and scaffold thrombosis. (ClinicalTrials.gov: NCT02004730)
Abbreviations
ARC: Academic Research Consortium
BVS: bioresorbable vascular scaffold(s)
CABG: coronary artery bypass grafting
CAD: coronary artery disease
DAPT: dual antiplatelet therapy
DES: drug-eluting stent
DOCE: device-oriented composite endpoint
IVUS: intravascular ultrasound
OCT: optical coherence tomography
PCI: percutaneous coronary intervention
POCE: patient-oriented composite endpoint
QCA: quantitative coronary angiography
SICI-GISE: Italian Society of Interventional Cardiology
SPECT: single photon emission computed tomography
ST: scaffold thrombosis
TLR: target lesion revascularisation
Introduction
Bioresorbable scaffolds (BRS) are a breakthrough technology that may improve the outcomes of patients undergoing percutaneous coronary intervention (PCI) by avoiding the well-known drawbacks of metallic stents. The Absorb everolimus-eluting bioresorbable vascular scaffold (BVS) (Abbott Vascular, Santa Clara, CA, USA) showed favourable clinical outcomes in initial randomised trials1,2 and registries3,4. However, an issue emerged regarding higher than expected scaffold thrombosis (ST) rates4,5. Of note, the potential advantages of BVS, namely the absence of very late ST and permanent vessel caging together with facilitation of future revascularisations, should be maximised in patients with diffuse coronary artery disease (CAD), where drug-eluting stents (DES) have their weaknesses6.
We designed the present study to evaluate the outcomes of the Absorb BVS in patients with long coronary lesions and/or multivessel CAD (Figure 1). We pre-specified the implantation technique to be applied throughout the study7.
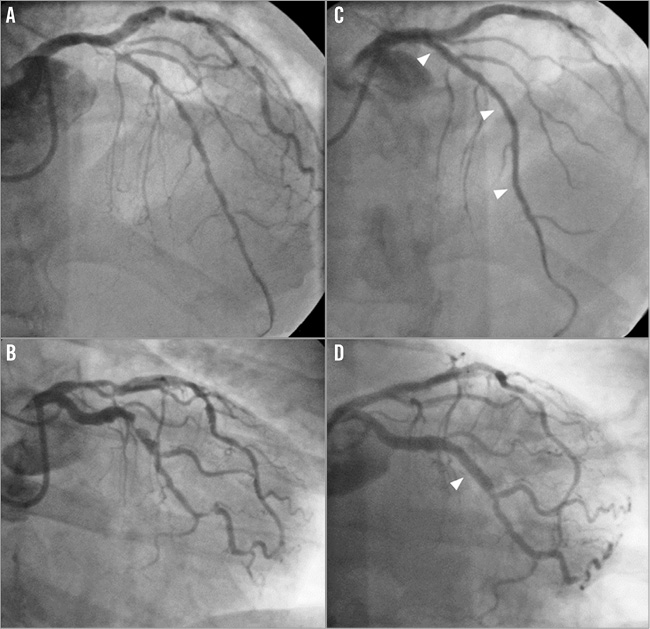
Figure 1. Typical example of coronary angiography of a patient enrolled in the registry. Baseline left coronary angiography of a patient showing diffuse disease of the left anterior descending artery (A) and severe stenosis of the circumflex artery (B). Final angiography after deployment of three scaffolds (arrowheads) in the left anterior descending artery (C) and of one scaffold in the circumflex (D).
Methods
The design of the IT-DISAPPEARS registry has been described previously8. In summary, IT-DISAPPEARS was a multicentre, prospective registry promoted by the Italian Society of Interventional Cardiology (SICI-GISE) (ClinicalTrials.gov number, NCT02004730). The institutional review board of each participating centre approved the study protocol.
All data were entered in a web-based electronic case report form (CRF) according to good clinical practice standards.
Almost all of the CRFs (98%) have been monitored by the Clinical Research Organisation (CRO) (Airtel SRL) that also built the e-CRF. Independent study monitors together with the investigators performed the data entry and verified the CRFs on-site. Among 38 centres actively enrolling, six centres enrolled less than six patients (totalling 21 cases [2%] out of 1,002). The CRFs of these cases were monitored only “remotely” by the CRO.
A clinical events committee adjudicated all adverse events with the specific focus of distinguishing the events related to the BVS from those not related to the BVS. A data and safety monitoring committee reviewed outcome data periodically. The study complies with the Declaration of Helsinki.
STUDY PATIENTS
Patients 18 years of age or older with: 1) evidence of myocardial ischaemia at stress echocardiography/myocardial single photon emission computed tomography (SPECT)/exercise test, or 2) unstable angina/non-STEMI, or 3) STEMI with de novo culprit lesion undergoing PCI for diffuse disease of one coronary vessel (lesion length ≥24 mm) and/or disease of at least two different coronary vessels were eligible for enrolment8. All patients provided written informed consent.
PROCEDURAL TECHNIQUE
All patients not on chronic aspirin treatment received a loading dose of ≥250 mg of aspirin within 24 hours before the procedure. A loading dose of a P2Y12 receptor antagonist was administered before the procedure or within one hour after the procedure. Other medications were administered according to current guidelines9-11. The use of quantitative coronary angiography (QCA), optical coherence tomography (OCT) or intravascular ultrasound (IVUS) to assess reference vessel diameter and lesion length, as well as to guide optimal scaffold implantation was recommended. This was part of a pre-specified technique for scaffold implantation that was published upfront and mandated7. After implantation, high-pressure post-dilatation with non-compliant (NC) balloons was recommended to achieve a residual stenosis ≤10%. The scaffold used in the study was the Absorb; starting from July 2015, the Absorb GT1 was also used according to local availability of the device. Available diameters and lengths were 2.5, 3.0 and 3.5 mm, and 8, 12, 18, 23 and 28 mm, respectively. Because of economic restraints and lack of specific scaffold sizes, the use of metallic stents was not forbidden. However, implantation of metallic stents and BVS in the same lesion and vessel was strongly discouraged. Among metallic DES, use of everolimus-eluting stents was recommended. Dual antiplatelet therapy was recommended for one year, aspirin lifelong.
Patients are followed up with ambulatory visits or telephone contact at 30 days, six months, one year and then yearly.
STUDY ENDPOINTS
The primary endpoint was the device-oriented composite endpoint (DOCE) of cardiac death, target vessel-related myocardial infarction (MI), and ischaemia-driven target lesion revascularisation (TLR) at one year. The secondary endpoints were: 1) the patient-oriented composite endpoint (POCE) of all-cause mortality, all MI and all revascularisations at one year; 2) the single components of the POCE, and 3) scaffold thrombosis (ST) at one year. Endpoint definitions follow the criteria of the Academic Research Consortium (ARC)12, and the third universal definition of MI13.
STATISTICAL ANALYSIS
Categorical variables are described as counts and percentages and compared using Pearson’s chi-square test or Fisher’s exact test. Continuous variables are described as mean±SD or as median and interquartile range (IQR) and compared using the t-test or Mann-Whitney test, as appropriate. Given that this is an observational registry, we relied on confidence interval profiling for sample size justification, without proceeding with formal power analysis. Accordingly, we computed that a target sample of 1,000 patients would enable the computation of reasonably precise 95% confidence intervals. Specifically, assuming a 4.2% MACE rate at one year (in keeping with ABSORB EXTEND data), confidence intervals computed with the adjusted Wald method would be 3.1% to 5.6% for a 1,000-patient sample (point estimate 42/1,000 [4.2%]). Events at one year will be presented as cumulative incidences.
A two-sided p-value of less than 0.05 was considered to indicate statistical significance. All statistical analyses were performed with the use of NCSS 11 software (NCSS LLC, Kaysville, UT, USA).
Results
PATIENT POPULATION
Between November 2014 and January 2016, we enrolled 1,002 patients, i.e., 5.2% of the overall PCI volume in the enrolling centres (N: 18,951 patients during the enrolment period). Clinical presentation was an ACS in 59.8% of patients, including STEMI in 21.8% (Table 1). One-, two-, three-vessel disease was present in 43.1%, 37.1%, and 19.8% of patients, respectively (Table 2).
PROCEDURAL FEATURES
The average number of lesions was 3.2±1.3 among patients undergoing multivessel BVS implantation. The average number of BVS implanted per patient was 2.0±1.0 (range 1-8), for a total BVS length of 47±22 mm. The percentage of patients receiving more than one BVS was 53.6%, and 48.2% of them required implantation of BVS in overlap/juxtaposition. At least one metallic stent was used in 41.9% of patients, mainly in shorter and distal lesions and not together with a BVS in the same lesion. The main reasons for DES implantation were budget constraints and availability of BVS sizes (both for small vessels [<2.5 mm] and for large vessels [>3.75 mm]). Multivessel BVS implantation was performed in 19.6% of patients. All patients were discharged on dual antiplatelet therapy (DAPT) (Table 2).
In total, 1,415 coronary lesions were treated with BVS with a total of 2,040 BVS implanted (Table 3). Predilatation and post-dilatation were performed in 98.0% and 96.5% of cases, respectively, using at least one NC balloon in 89.4%, with a balloon:scaffold ratio of 1.06±0.09.
PRIMARY AND SECONDARY ENDPOINTS
In-hospital and one-year adverse events are summarised in Figure 2. In-hospital MI occurred in 3.4% of patients, consisting of 2.8% periprocedural type 4a MI and 0.6% due to definite ST. The timing of ST was intraprocedural in two patients, and after eight hours, three days, 10 days, and 14 days in the remaining four patients. All patients underwent emergent coronary angiography and ischaemia-driven TLR; one additional ischaemia-driven TLR was performed before discharge in a patient with unstable angina and angiographic evidence of subocclusion of a side branch (Table 4).
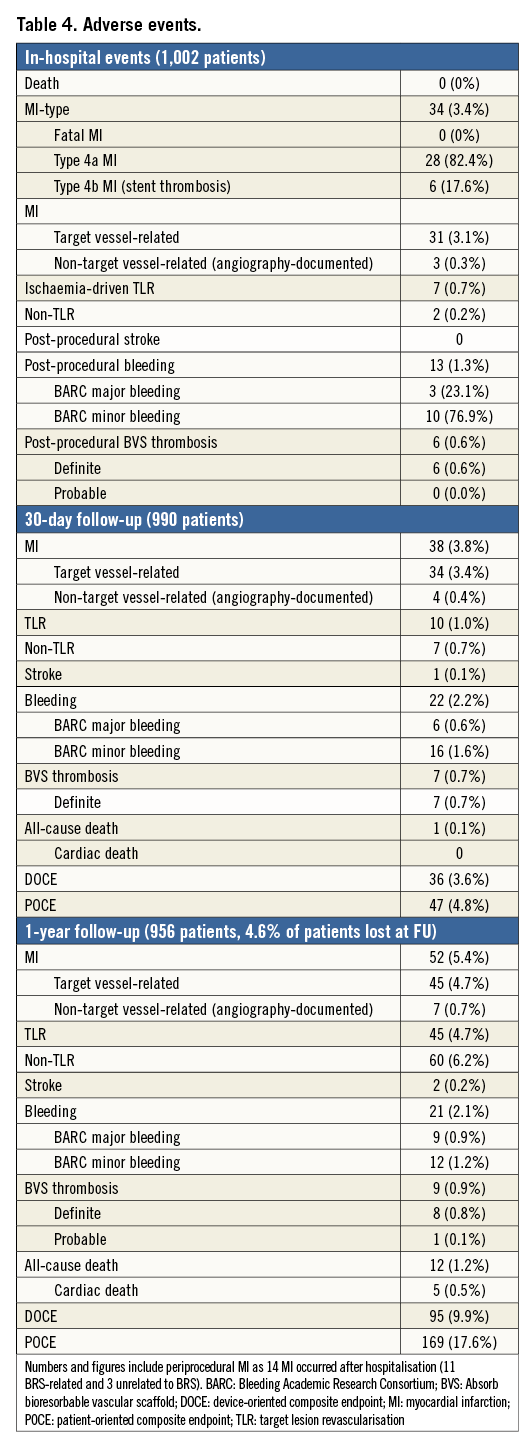
One-year follow-up was available in 956 patients (95.4%). The rate of DOCE was 9.9% (95 patients). Cardiac death occurred in five patients (0.5%), while target vessel MI and TLR each occurred in 45 (4.7%) patients. The one-year rates of all-cause death, non-fatal MI, and any revascularisation were 1.2%, 5.4%, and 10.9%, respectively (Figure 2). The rate of TVR was 4.7%, as all the TVR were actually TLR. Indeed, the rate of target vessel failure (the composite of cardiac death, target vessel myocardial infarction, and ischaemia-driven target vessel revascularisation) was 9.9%, identical to the DOCE. The rate of non-TLR/TVR was 6.2%, and thus the rate of any revascularisation was 10.9%. The rate of definite/probable scaffold thrombosis was 0.9%, with only one case of “probable” ST (0.1%) (Table 4).
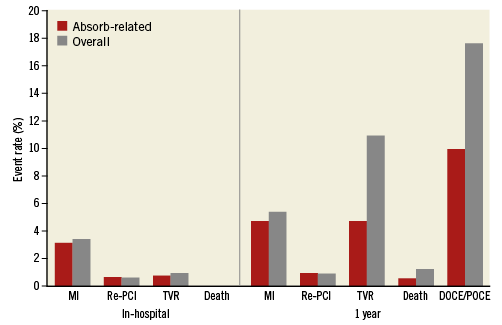
Figure 2. In-hospital and 1-year cumulative rates of major adverse events. Histogram showing the rates of adverse events occurring during the index hospitalisation and at one-year follow-up; red bars indicate scaffold-related events, grey bars indicate overall events. DOCE: device-oriented composite endpoint; MI: myocardial infarction (including periprocedural); PCI: percutaneous coronary intervention; POCE: patient-oriented composite endpoint; ST: stent thrombosis
Discussion
The present study is the first large prospective registry to evaluate the clinical outcomes of Absorb BVS in patients with long coronary lesions and/or multivessel CAD.
Results can be summarised as follows: 1) the one-year rate of DOCE was 9.9%; 2) the one-year rate of target vessel MI and TLR was 4.7% in both; 3) the one-year rate of definite/probable ST was 0.9%.
The favourable initial reports on the clinical outcomes of the Absorb1,2,14,15 spurred on its use in more complex clinical and coronary anatomy conditions16,17. Indeed, the first “real-world” prospective registries of Absorb showed acceptable outcomes, but reported a disturbing signal regarding an unpredicted high ST rate3,4,18, possibly related to an inadequate implantation technique19.
Our study has two major distinctive features compared to all other contemporary large multicentre registries: 1) all patients had diffuse CAD by inclusion criteria, with almost 100% ACC/AHA type B2/C lesions, as compared to 40% to 51% in previous all-comers BVS registries4,19,20; 2) a carefully predefined and previously published implantation technique was mandated in all patients. This led to a post-dilatation rate of 96.5%, as compared to a rate ranging between 40% and 72% in other BVS registries4,20.
Our patients were younger (average 60±10 years), with fewer comorbidities than the average PCI population, with a higher rate of multivessel CAD (57.1%) as compared to most BVS registries. In the present registry, the average lesion length was 28.5±13.1 mm, about 10 mm longer than that reported by other BVS registries4,20. Accordingly, the overall BVS length of 47±22 mm and the 48.2% of overlapping BVS are by far the highest ever-reported length and rate in registries. The encouraging early clinical outcomes of our complex patient population corroborate the preliminary findings of the long lesion subgroup analysis of the GHOST-EU registry20 and of a small propensity-matched comparison between BVS and DES in long lesions, showing similar DOCE rates at one year21.
Although a recent meta-analysis failed to identify an association between the percentage of predilation or post-dilation and the risk of 30-day ST in the initial BVS trials and registries22, a causal role of inadequate implantation technique, with subsequent scaffold underexpansion and malapposition, has been postulated as a possible risk of scaffold failure, along with inadequate antiplatelet therapy23. Indeed, the recently published PSP score, although having poor discrimination and calibration, at one-year follow-up independently predicted DOCE24. This is quite reassuring, considering that, compared to the GHOST-EU registry from which the PSP score was created and validated, our data were generated prospectively, an independent clinical events committee adjudicated the events, and data about balloon sizes and pressures were fully available.
Recently, the two-year results of the ABSORB III trial were presented (at the 2017 American College of Cardiology Congress), reporting significantly higher TLF rates with Absorb vs. XIENCE (Abbott Vascular) (11.0% vs. 7.9%, p=0.03). In ABSORB III, vessels with a reference diameter <2.25 mm accounted for 19% of the entire population, while in IT-DISAPPEARS we excluded lesions in vessels with a reference diameter <2.5 mm. In addition, the post-dilatation rate was 66% in ABSORB III vs. 97% in IT-DISAPPEARS. The recently published AIDA trial25 added further evidence on the higher risk of ST with the Absorb (3.1% of definite/probable ST versus 0.6% with DES) but also on the equivalence of the Absorb with an everolimus-eluting stent in terms of the composite endpoints.
In conclusion, IT-DISAPPEARS can be regarded as a prospective demonstration that, when a careful technique is used, Absorb implantation can be associated with an excellent safety and efficacy profile, even in patients with high lesion complexity.
Limitations
Consecutiveness of enrolment was not monitored, thus we cannot rule out a certain degree of selection bias, possibly affecting the subsequent rate of procedural success. Second, although we recommended limiting the use of metallic DES, these were implanted in some patients. The main reasons for implanting a DES were: budget constraints and availability of BVS sizes. However, to overcome this issue, all events were carefully adjudicated to estimate the true BVS-related events. Metallic DES were used with BVS in the same lesion in only 4% of all BVS-treated lesions. Data concerning the features of treated lesions are reported according to an on-line QCA evaluation made by every single operator during implantation. Our primary endpoint was at one year: more efficacy/safety information will come from the analysis of later follow-ups, i.e., when the resorption process will be at a more advanced stage.
Conclusions
This is the first study specifically designed to investigate the Absorb technology in patients with a high atherosclerotic burden and multivessel disease. The adherence to a mandatory pre-specified implantation technique seemed able to minimise the risk of the device-oriented composite endpoint and scaffold thrombosis. A longer follow-up will further clarify the impact of the implantation technique on outcomes.
| Impact on daily practice The primary outcome of the IT-DISAPPEARS registry supports the routine application of a careful implantation technique that includes lesion assessment, preparation, and scaffold post-dilation. |
Funding
The Italian Society of Interventional Cardiology (SICI-GISE) (ClinicalTrials.gov: NCT02004730) has promoted this study. Abbott Vascular provided an unrestricted grant to SICI-GISE.
Conflict of interest statement
The authors have no conflicts of interest to declare.

