
Abstract
Aims: It has become apparent that, in comparison to metallic stents, bioresorbable vascular scaffolds (BVS) require specific implantation techniques. The aim of this study was to investigate outcomes following BVS implantation using a dedicated strategy for optimal deployment.
Methods and results: Four hundred consecutive lesions (264 patients) treated with the Absorb BVS were analysed. All procedures were performed based on the following principles: 1) aggressive lesion preparation; 2) high-pressure post-dilation; and 3) a low threshold for intravascular imaging. The majority of target lesions (74.8%) were type B2 or C lesions. Predilation (97.3%) and post-dilation (99.8%) were performed in almost all cases. The mean post-dilation pressure was 21±5 atm, and the total scaffold length per patient was 53.2±32.5 mm. Intravascular imaging was performed in the majority of cases (85.8%) and, when utilised after post-dilatation, a further intervention was required in 24.5% of lesions. The cumulative target lesion failure rates were 7.9% at one year and 11.6% at two years. Definite/probable scaffold thrombosis occurred in three patients (1.2% at one and two years).
Conclusions: Clinical outcomes following implantation of current-generation BVS, in a real-world population with a high prevalence of complex lesions, were acceptable when utilising our optimised implantation strategy.
Abbreviations
BVS: bioresorbable vascular scaffold
CABG: coronary artery bypass grafting
DAPT: dual antiplatelet therapy
DES: drug-eluting stent
LAD: left anterior descending artery
MI: myocardial infarction
NC: non-compliant
PCI: percutaneous coronary intervention
QCA: quantitative coronary angiography
ST: scaffold thrombosis
TLF: target lesion failure
TLR: target lesion revascularisation
TVR: target vessel revascularisation
Introduction
Bioresorbable vascular scaffolds (BVS) have become an attractive option in the field of percutaneous coronary intervention (PCI) due to the potential advantages associated with complete resorption within a few years1. Recent randomised trials have indicated non-inferiority in one-year outcomes between current BVS and contemporary metallic drug-eluting stents (DES) when used to treat simple lesions2-6. Furthermore, real-world registries have reported acceptable outcomes even in complex lesions7,8; however, concerns have been raised regarding a higher risk of scaffold thrombosis as compared to metallic DES, with limited data being available regarding long-term outcomes, especially in complex lesions.
Additionally, currently available BVS have several limitations including reduced radial force and increased strut thickness when compared to their metallic counterparts9,10. Dedicated implantation techniques are therefore needed to try to overcome the characteristics of current-generation BVS11, reduce the risk of early scaffold thrombosis and yield favourable long-term outcomes. We investigated the clinical outcomes utilising a dedicated strategy for optimal BVS deployment in a real-world cohort with a high prevalence of complex lesions.
Methods
SUBJECTS
Data were examined from all lesions treated with Absorb™ BVS (Abbott Vascular, Santa Clara, CA, USA) at two high-volume centres in Milan, Italy, between May 2012 and August 2015 (400 lesions in 264 patients). All patients provided informed consent for both the procedure and subsequent data collection and analysis. Clinical follow-up was performed by routine clinical visits or telephonic interview.
PROCEDURE AND MEDICATIONS
The PCI strategy was dependent upon individual operators; however, all procedures were performed based on the following principles:
PREDILATION AND LESION PREPARATION
Lesion preparation was generally performed with non-compliant balloons (sized 1:1 with the vessel diameter and implanted scaffold). The operator confirmed complete expansion without indentation to ensure adequate lesion preparation prior to BVS implantation. In cases of inadequate lesion preparation with non-compliant balloons, adjunctive devices including scoring and cutting balloons or rotational atherectomy were utilised.
SCAFFOLD IMPLANTATION
Inflation pressure was slowly increased (2 atm per 5 seconds) up to 8-10 atm, followed by a prolonged inflation (more than 30 seconds) to complete expansion. When implanting BVS in lesions that required multiple BVS, a “scaffold to scaffold” implantation technique was adopted to minimise overlap, which was achieved by placing the balloon marker of the proximal scaffold just before the scaffold marker of the distal scaffold.
POST-DILATION
Post-dilation was performed with non-oversized non-compliant balloons (scaffold/balloon diameter 1:1) at high pressures (≥20 atm) to ensure optimal scaffold expansion. When further post-dilation was required, either higher pressures with the same balloon, or a different balloon with diameter equal to scaffold size + a maximum of 0.5 mm were used.
INTRAVASCULAR IMAGING
Operators had a low threshold for baseline intravascular imaging (intravascular ultrasound or optical coherence tomography) to confirm vessel/lumen diameter, especially in angiographically large (>3.75 mm) or small vessels (<2.5 mm).
Whenever possible, intravascular imaging was used at the end of the procedure to confirm optimal scaffold expansion, exclude edge dissections and malapposition. Underexpansion and/or malapposition was aggressively managed with additional post-dilation, and the AVIO (Angiography vs. IVUS Optimisation) criteria were used to guide target scaffold area12.
All patients received dual antiplatelet therapy (DAPT) (aspirin and clopidogrel) for at least 12 months following BVS implantation. In patients with complex lesions including bifurcations treated with double stenting and/or multiple lesions treated with BVS, the use of ticagrelor or prasugrel as a substitute for clopidogrel was preferred for the first one to three months, followed by de-escalation to clopidogrel to complete a minimum of 12 months DAPT.
ENDPOINTS
The primary endpoint was target lesion failure (TLF), defined as a composite of cardiac death, target vessel myocardial infarction (MI), or clinically driven target lesion revascularisation (TLR). Other endpoints included all-cause mortality, target vessel revascularisation (TVR), and definite/probable scaffold thrombosis (ST).
DEFINITIONS
MI was defined as the presence of new pathological Q-waves on an electrocardiogram, or an increase in creatinine kinase-myocardial band level to >5x the upper limit of the normal range13. TLR was defined as repeat PCI or coronary artery bypass grafting (CABG) for the target segment or in the adjacent proximal or distal 5 mm. TVR was defined as repeat PCI or CABG in the target vessel. ST was defined according to the Academic Research Consortium criteria14.
The SYNergy between PCI with TAXUS and Cardiac Surgery (SYNTAX) score was calculated for patients without previous CABG15. Quantitative coronary angiography (QCA) analyses were performed using a validated edge detection system (CMS, version 5.2; Medis medical imaging systems bv, Leiden, The Netherlands). The following QCA parameters were measured: reference vessel diameter, minimal lumen diameter, percentage diameter stenosis, and acute gain.
Severe calcification was defined as radiopacities noted at the stenosis site without cardiac motion prior to contrast injection from at least two angiographic views.
STATISTICAL ANALYSIS
Data are presented as mean±SD or median (interquartile range). Categorical variables are expressed as numbers and percentages. Continuous data were compared using unpaired t-tests. Categorical data were compared using the chi-square or Fisher’s exact test. Cumulative event rates were calculated using the Kaplan-Meier method. All analyses were performed using SPSS, Version 21.0 software package (IBM Corp., Armonk, NY, USA).
Results
The baseline patient characteristics of all patients are shown in Table 1. In the overall cohort, the rate of diabetes was 26.1%, prior PCI and MI 43.9% and 27.3%, respectively. ST-segment elevation MI (STEMI) or NSTEMI as a reason for PCI was only 1.9%. The mean SYNTAX score of the cohort was 17.1±10.4.
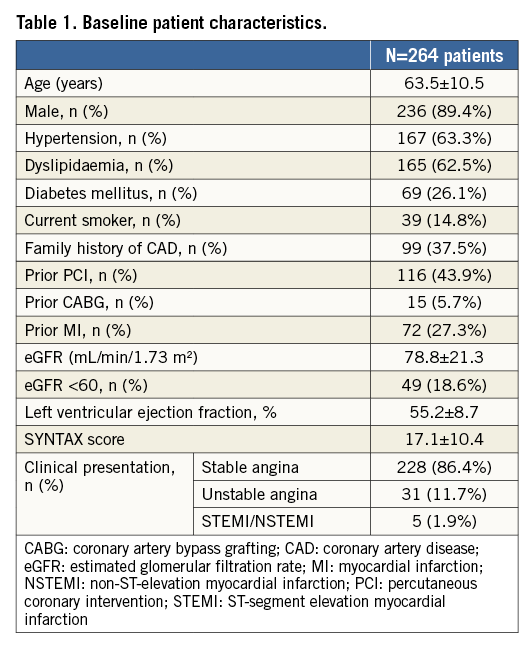
Table 2 describes lesion and procedural characteristics. Out of 400 lesions, the majority (74.8%) were type B2 or C according to ACC/AHA classification, 46.8% were bifurcations (side branch ≥2.25 mm on visual assessment), and 22.5% severely calcified.
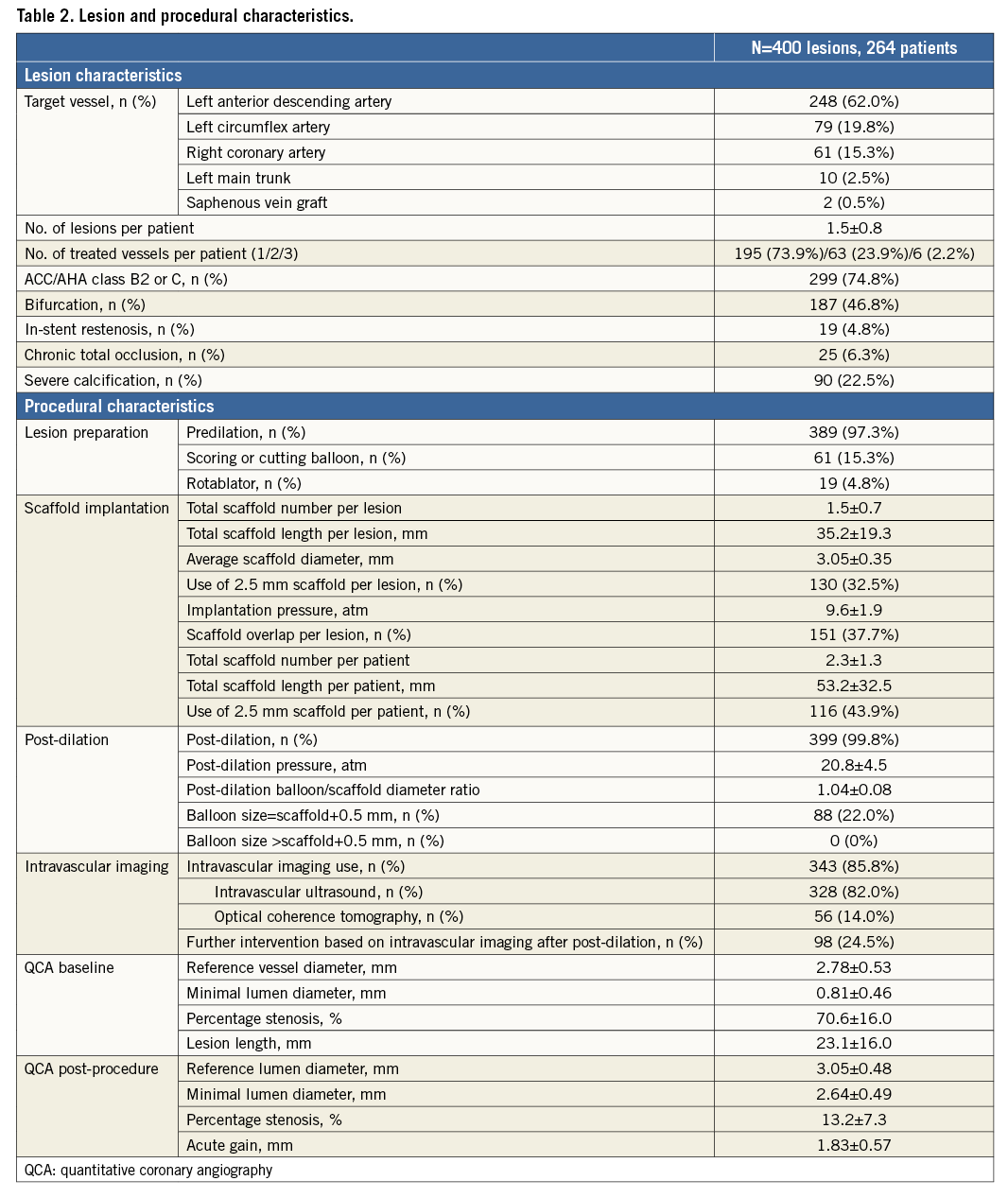
The mean scaffold diameter was 3.05±0.35 mm and total scaffold length 35.2±19.3 mm per lesion. The total scaffold length per patient was 53.2±32.5 mm, and 43.9% of patients received at least one 2.5 mm BVS.
Predilation (97.3%) and post-dilation (99.8%) were performed in almost all cases. Adjunctive devices for lesion preparation (scoring or cutting balloons, or rotational atherectomy) were utilised in about 20% of cases. The mean post-dilation pressure was 21±5 atm and the balloon/scaffold ratio was 1.04±0.08.
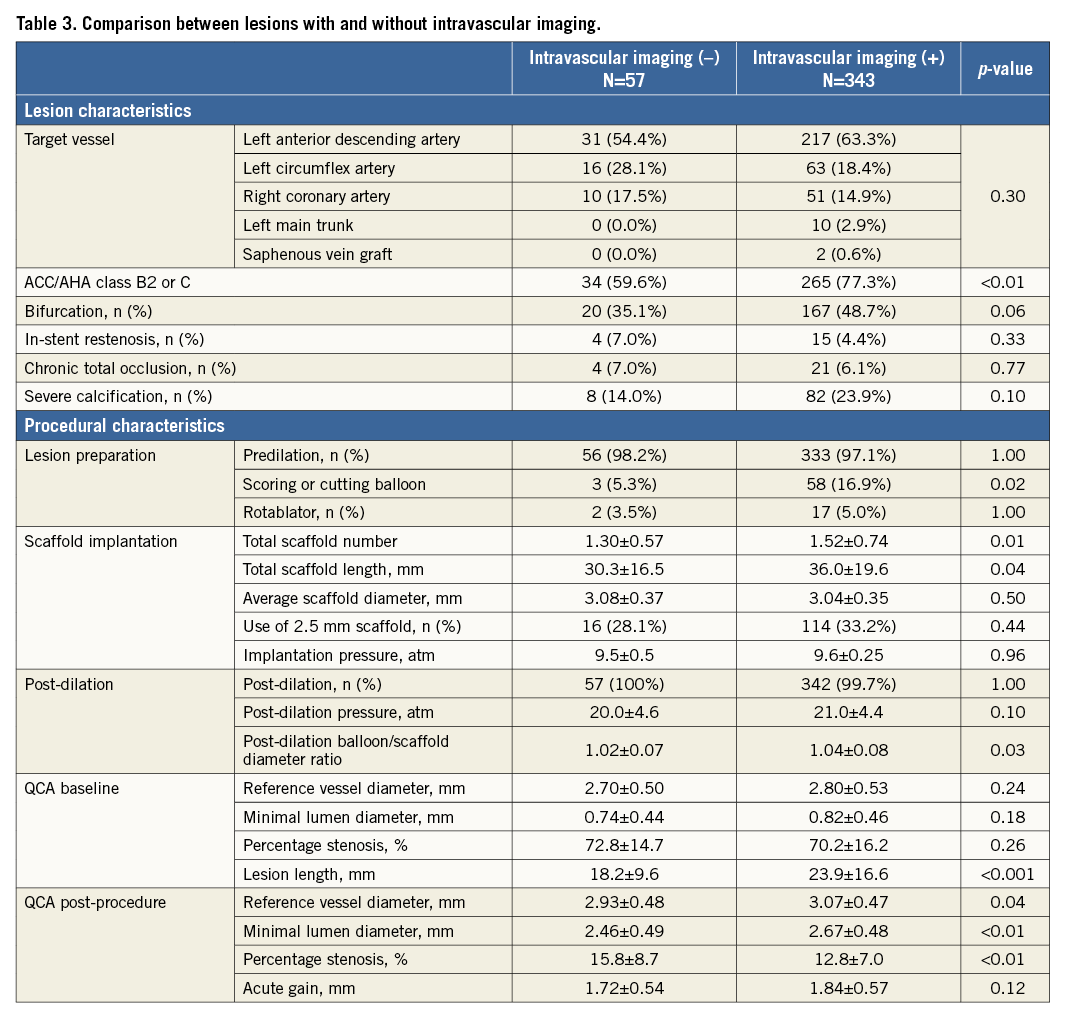
Intravascular imaging (intravascular ultrasound or optical coherence tomography) was performed in the majority of cases (85.8%). A total of 24.5% of lesions required further intervention after post-dilation based on intravascular imaging findings. The main reason for additional interventions was scaffold underexpansion in 82 lesions, malapposition in 11 lesions, edge dissection in four lesions, and incomplete lesion coverage in one lesion. When comparing the lesions with or without intravascular imaging use (Table 3), lesion/total scaffold lengths were shorter and less complex with lower rates of type B2/C lesions, if intravascular imaging was not used. In addition, the post-dilation balloon/scaffold diameter ratio was lower and final residual percentage stenosis larger without intravascular imaging guidance.
In 46.6% of patients, ticagrelor (103/264, 39.0%) or prasugrel (20/264, 7.6%) was administered as a substitute for clopidogrel at discharge.
The median patient follow-up was 544 days (interquartile range 228-834 days). The cumulative TLF rates according to Kaplan-Meier analysis were 7.9% at one year and 11.6% at two years (Figure 1A, Table 4). The rates of cardiac death were 1.3% at one year and 2.0% at two years (Figure 1B), target vessel MI 1.8% at both one and two years (Figure 1C), and TLR 6.6% at one year and 10.4% at two years (Figure 1D).
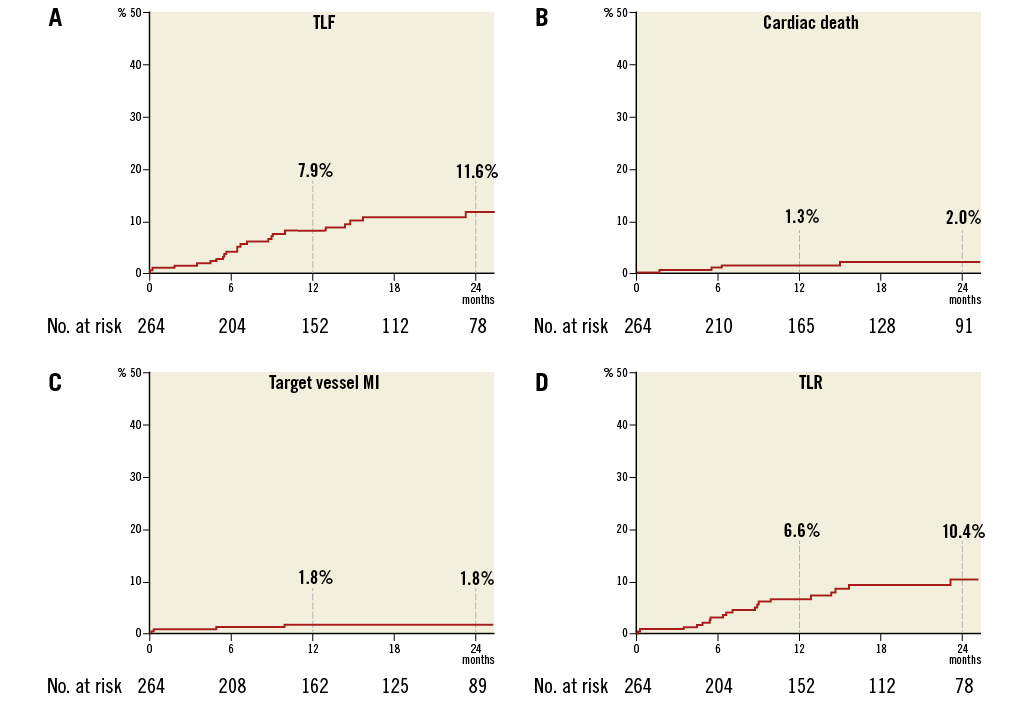
Figure 1. Kaplan-Meier curves. A) Target lesion failure. B) Cardiac death. C) Target vessel myocardial infarction. D) Target lesion revascularisation.
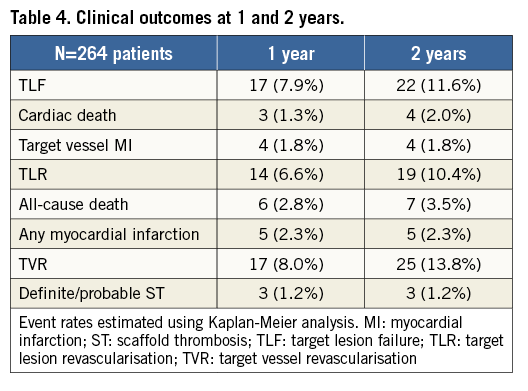
Definite or probable ST occurred in three patients (1.2% at one year, remaining the same at two years). One patient developed acute scaffold thrombosis after urgent BVS implantation (prox. LAD) in the setting of an ST-segment elevation acute myocardial infarction. Another patient developed subacute ST at day three after two continuous BVS were deployed in a proximal LAD, after which edge dissections were observed, but bail-out stenting was not performed due to the good distal flow. The third patient developed late ST after 146 days due to the premature discontinuation of clopidogrel after two months following three implanted BVS in diffuse LAD disease. ST cases were managed successfully by repeat PCI with drug-eluting stent implantation, and all patients survived to discharge.
Discussion
In this real-world cohort of BVS implantation, 1) a high prevalence of complex lesions was treated; 2) dedicated implantation techniques were consistently utilised including the almost universal performance of predilation, high-pressure post-dilation, and intravascular imaging; and 3) considering the complex lesion subset, clinical outcomes were good.
Recent randomised trials have demonstrated comparable clinical outcomes following BVS implantation when compared to current-generation drug-eluting stents in relatively simple lesions2-4,6. Furthermore, acceptable results have been reported in several real-world registries, even in complex lesions7,8,16,17. Regarding BVS implantation in complex lesions, our data are unprecedented. The rates of type B2/C lesions were 68.7% in the largest randomised trial (ABSORB III)2, 53.5% in the largest real-world registry (GHOST-EU registry)7, and 74.8% in ours. Notably, bifurcation lesions were excluded in ABSORB III, and only present in 23.1% of lesions in GHOST-EU, in contrast to 46.8% in our cohort. Additionally, the mean total scaffold length was 20.5±7.2 mm per lesion in ABSORB III (one lesion treated in almost all patients), 32.6±23.0 mm per patient in GHOST-EU, and 53.2±32.5 mm in our registry.
The heterogeneous outcomes reported by the studies of current BVS may be due to the differing strategies used for implantation11. Obtaining the best results following current BVS implantation may be dependent on the following scaffold optimisation techniques:
PREDILATION AND LESION PREPARATION
BVS seem to have greater acute recoil than metallic stents18, and inadequate lesion preparation may correlate with underexpansion18,19. Adequate lesion preparation is required not merely for scaffold delivery but more importantly for optimal and symmetrical scaffold expansion. Therefore, predilation should have a more important role with current BVS than metallic stents, and a liberal usage of adjunctive devices is encouraged.
POST-DILATION
Recent trials have demonstrated that acute lumen gain is lower for current BVS than for metallic stents, with similar pressures even in a simple lesion subset2-4. Caiazzo et al indicated that studies reporting high post-dilation rates (over 90%) and pressures (over 20 atm) were associated with lower rates of ST17, which might suggest the importance of high-pressure post-dilation to achieve optimal expansion and better clinical outcomes4,11,17,19-21. However, overexpansion can cause scaffold fracture10; therefore, judicious NC balloon sizing is important. In this study, the post-dilation balloon/scaffold diameter ratio was 1.04±0.08, with 21±5 atm.
INTRAVASCULAR IMAGING
Due to the overexpansion limitations of BVS and to prevent increased footprint, intracoronary imaging is important for vessel sizing in angiographically ambiguous cases such as diffuse disease. The fact that BVS underexpansion is more common with current BVS stresses the importance of intravascular imaging. Despite containing more complex lesions, the final residual percentage stenosis of lesions was lower if intravascular imaging was used to guide implantation in our cohort. In addition, the need for more aggressive predilation and post-dilation to obtain optimal scaffold expansion may increase the incidence of edge dissections. In a case series of scaffold thrombosis in BVS22, underexpansion, incomplete lesion coverage, and malapposition were shown to be the main causes of scaffold thrombosis. Therefore, intravascular imaging is important, especially at the end of the procedure, to confirm adequate expansion and to evaluate the presence of edge injuries or malapposition. This may lead to better clinical outcomes11,21,23. In our data, a total of 24.5% of lesions required further intervention after post-dilation based on intravascular imaging findings.
Although the importance of the above strategies has already been indicated7,24, few reports are available regarding outcomes in BVS implanted with a specific scaffold optimisation strategy. The performance rates of post-dilation and intravascular imaging were only 65.5% and 11.2% in the ABSORB III trial2, and 49% and 14.4% in the GHOST-EU registry7, but much higher in our registry (99.8% and 85.8%). Considering the complex lesions treated in our population, we were still able to obtain significant acute gain after deployment (1.83±0.57 mm in our data, and 1.45±0.45 mm in ABSORB III), with acceptable clinical outcomes (TLF at one year was 7.9% in our data, vs. 7.8% in ABSORB III, 4.4% [six months] in GHOST-EU). These results are likely to reflect our scaffold deployment strategy. On the flip side, longer procedure and fluoroscopy times with greater contrast use and higher procedural costs might be inevitable25.
Limitations
This study had several limitations. Firstly, this was a single-arm observational study in which optimal BVS implantation was performed from the beginning, and therefore we could not compare clinical outcomes following BVS implantation without an optimal implantation strategy. Secondly, the study population was relatively small in number. Thirdly, the number of patients undergoing PCI for acute coronary syndromes was low (13.6%), and therefore our results cannot be extrapolated to this subset of patients. Finally, it is impossible to quantify the impact of using more potent antiplatelet agents on the prevention of early scaffold thrombosis.
Conclusions
One- and two-year clinical outcomes following implantation of current-generation BVS, in a real-world population with a high prevalence of complex lesions, were acceptable when utilising a consistent optimal implantation strategy. It is imperative that we acknowledge that, in comparison to metallic DES, current-generation BVS are less forgiving to suboptimal implantation and may require a dedicated strategy to ensure good clinical outcomes.
| Impact on daily practice Although the importance of the above strategy has already been mentioned, few reports are available regarding outcomes of BVS implanted with a specific scaffold optimisation strategy. In this real-world cohort of BVS implantation, clinical outcomes were good even considering the complex lesion subset. These results may suggest the importance of dedicated implantation techniques, including predilation, high-pressure post-dilation and intravascular imaging. |
Funding
A. Tanaka was supported by a research fellowship programme of the Uehara Memorial Foundation and received a grant from the Fukuda Memorial Foundation for Medical Research.
Conflict of interest statement
A. Latib serves on the advisory board for Medtronic. The other authors have no conflicts of interest to declare.

