
Abstract
Aims: To compare the feasibility, procedural and clinical outcomes after implantation of bioresorbable vascular scaffolds (BVS) in patients with calcified lesions.
Methods and results: We assessed the feasibility of BVS implantation and procedural outcomes in patients with and without calcific lesions. The primary outcome was angiographic and procedural success. Secondary outcomes included major adverse cardiovascular events (MACE). Of 163 patients, 62 (38%) had calcified lesions. Patients with calcific lesions had a higher prevalence of diabetes (35.5% vs. 22.8%, p=0.078) and chronic kidney disease (31.1% vs. 13.9%, p=0.008), and higher SYNTAX scores (18.9±9.7 vs. 15.1±9.0, p=0.017). Calcific lesions required longer procedures (126.4±39.8 vs. 106.9±37.1 min, p=0.015), more frequent use of dedicated devices and IVUS. Acute gain (1.83±0.6 vs. 1.86±0.6, p=0.732) and angiographic success were similar (98% non-calcific vs. 95.2% calcific, p=0.369), whereas procedural success was reduced in patients with calcific lesions (94.1% vs. 83.9%, p=0.034) due to higher rates of periprocedural myocardial infarction (MI) (5% vs. 13.1%, p=0.067). During the median follow-up time of 14 months MACE rates (10.9% non-calcific vs. 12.9% calcific, plog-rank=0.546) were similar.
Conclusions: Treating calcific lesions with BVS is feasible with high angiographic success rates, at the expense of longer procedure times, aggressive lesion preparation and increased rates of periprocedural MI.
Introduction
Even though the safety profile of drug-eluting stents (DES) has improved with the introduction of thinner struts and biodegradable polymers1, late lumen loss, late stent thrombosis2 and permanent vessel caging are still haunting interventional practice. Bioresorbable vascular scaffolds (BVS) transiently support the vessel wall with a scaffold which will subsequently disappear. Good long-term outcomes (major adverse cardiovascular events [MACE] 3.4%) have been demonstrated in the five-year follow-up of the ABSORB cohort A3 involving 30 patients with single simple de novo lesions treated with BVS (Absorb v1.0; Abbott Vascular, Santa Clara, CA, USA). In the ABSORB II randomised controlled trial (maximum two de novo simple lesions)4, 335 patients treated with the Absorb v1.1 BVS were compared to 166 patients treated with an everolimus-eluting metallic stent (EES) (XIENCE; Abbott Vascular). Despite the lower post-implantation acute lumen gain observed in lesions treated with BVS, one-year target lesion failure was similar between the two groups (BVS vs. EES; 5% vs. 3%, p=0.35). In contrast to DES, despite the increase in neointima thickness from six months to two years, the luminal dimensions in vessels treated with BVS are not compromised5, suggesting late scaffold enlargement and expansive vessel remodelling.
The prevalence of coronary calcification (Agatston >0 on computed tomography) in the asymptomatic population is as high as 60% in males and 40% in females of different ethnicities6. Moderate/severe calcification was prevalent angiographically in 38% of lesions whereas intravascular ultrasound (IVUS) detected calcium in 73% of lesions7. Calcific lesions remain a challenge for the interventional cardiologist despite the advent of low-profile, non-compliant, high-pressure, bladed or scoring balloons. In tertiary centres, rotablation use is required in up to 5% of cases8. MACE rates after treatment of calcific lesions are higher compared to the general PCI population9, probably reflecting increased rates of incomplete revascularisation and more extensive coronary artery disease (CAD) in a population with a high comorbidity burden, particularly diabetes and chronic kidney disease (CKD). On some occasions, despite meticulous lesion preparation, optimal strut apposition is not possible10. The increased BVS strut thickness (leading to inferior deliverability) and reduced radial force (with time and expansion beyond nominal size) have raised concerns regarding the feasibility, performance and clinical outcomes of BVS in the treatment of calcific lesions. Use of BVS in patients with calcific lesions, however, would be of particular benefit as these patients tend to have more extensive disease requiring longer stents. Furthermore, even though restoration of vasomotion in a circumferentially calcified lesion is unlikely to occur unless an atherectomy strategy is employed, positive remodelling and partial vasomotion restoration would be expected in lesions with moderate non-circumferential calcification5.
To date, only anecdotal evidence exists in the literature11,12 regarding the feasibility of BVS use in calcific lesions. The present study presents the procedural feasibility and one-year clinical outcomes of patients with calcific lesions treated with BVS, and compares them to those of patients with non-calcific lesions.
Methods
Between May 2012 and May 2014, 163 patients were treated with the Absorb BVS v1.1 (Abbott Vascular) in two Italian centres (San Raffaele Scientific Institute and EMO GVM Centro Cuore Columbus, Milan, Italy). Written informed consent was obtained from all participants for the procedure, data collection and subsequent analysis and publication. IVUS was performed in 127 (77.9%) cases using the iLab™ Ultrasound Imaging System (Boston Scientific, Marlborough, MA, USA) and the OptiCross™ imaging catheter (Boston Scientific).
Of all patients treated with BVS, those with at least one calcified lesion (defined as calcium arc >90° on IVUS or at least moderate lesion calcification on angiography in the absence of IVUS) were classified as the “calcific group”, whereas patients with no calcific lesions were classified as the “non-calcific group”. Angiographically moderate/severe calcification was defined as radiopacities noted with or without cardiac motion before contrast injection, generally compromising both sides of the arterial lumen7,13. In the study by Mintz et al7, moderate calcification angiographically exhibited a mean calcification arc of 165° on IVUS (in the current study the mean calcium arc was 183.5°). The cut-off of 90° was selected, as in previous studies it has been demonstrated that a calcium arc >90° is associated with reduced stent expansion and increasing use of rotablation14,15.
All patients were pretreated with aspirin and clopidogrel or ticagrelor or prasugrel and instructed to carry on with dual antiplatelet therapy for at least one year. Quantitative coronary angiographic (QCA) measurements were performed offline using a validated edge detection system (CMS, version 5.2; Medis Medical Imaging Systems BV, Leiden, The Netherlands) by an experienced interventional cardiologist (TN) not otherwise involved in the current trial and its outcomes; minimal lumen diameter (MLD), reference vessel diameter (RVD), and percentage of diameter stenosis were measured at baseline.
The primary endpoints included angiographic success, procedural success and periprocedural myocardial infarction. Angiographic success was defined as a minimum stenosis diameter of <20% (by QCA), with TIMI flow grade 3 without occlusion of a significant side branch, flow-limiting dissection, distal embolisation or angiographic evidence of thrombus. Procedural success was defined as the composite endpoint of angiographic success without associated in-hospital major clinical complications (e.g., death, MI, stroke, emergency CABG)16.
Periprocedural MI (<48 hrs) post PCI was defined as per Table 1 in the paper by Vranckx et al17. Secondary endpoints included one-year MACE (defined as the composite of all-cause mortality, non-procedural myocardial infarction [MI] and any revascularisation) and one-year target lesion revascularisation (TLR: defined as repeat revascularisation within the stented segment, or within 5 mm from the stent edges). Follow-up MI was defined as per current guidelines18. Stent thrombosis was classified according to the Academic Research Consortium definition19.
Statistical analysis
All continuous variables were tested for normality using the Kolmogorov-Smirnov test. Data are presented as percentages, mean±standard deviation (SD) or median (interquartile range). Differences in proportions were tested with the chi-square test or Fisher’s exact test, and differences in continuous variables were tested with the Student’s t-test or Wilcoxon rank-sum test for parametric and non-metric variables, respectively. Cumulative event curves were generated using the Kaplan-Meier method.
Results
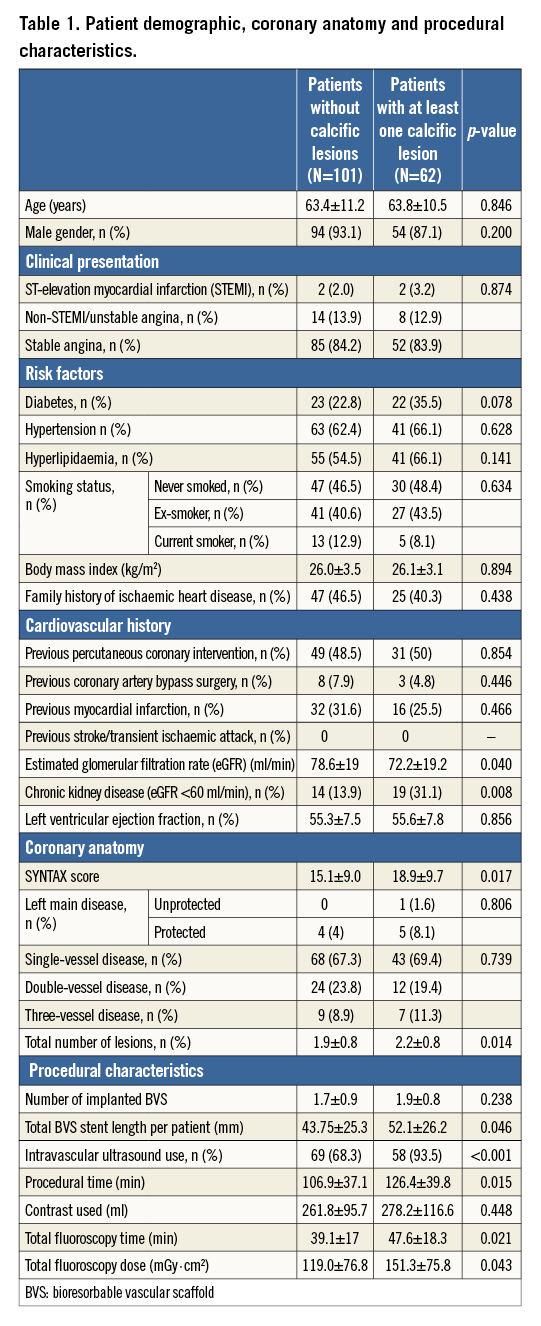
Out of 163 patients treated with BVS, 62 (38%) had at least one more than moderately calcified lesion. In the calcific group, the mean calcium arc was 183.5±105°. Mean age, male gender and clinical presentation were similar between the two groups (Table 1). Regarding risk factors, there was a significantly higher prevalence of CKD amongst patients with calcific lesions (31.1% vs. 13.9%, p=0.040) and a trend for a higher prevalence of diabetes (35.5% vs. 22.8%, p=0.078). Patients with calcific lesions had more complex coronary artery disease (SYNTAX score: 18.9±9.7 vs. 15.1±9.0, p=0.017).
LESION AND PROCEDURAL CHARACTERISTICS
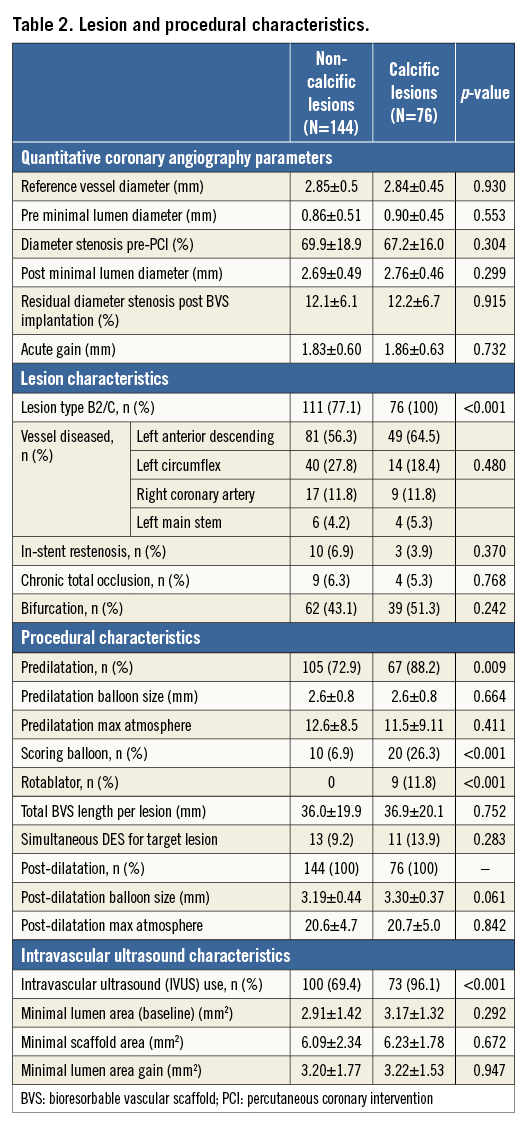
Lesion QCA parameters and diseased vessels were similar between calcific and non-calcific lesions (Table 2). All calcific lesions were classified as AHA/ACC B2/C versus 77.1% of the non-calcific ones (p<0.001). The use of predilatation (88.2% vs. 72.9%, p=0.009), scoring balloons (26.3% vs. 6.9%, p<0.001) and rotational atherectomy (11.8% vs. 0%, p<0.001) was significantly higher in calcific lesions. There was a higher use of IVUS in calcific lesions (96.1% vs. 69.4%, p<0.001) which facilitated the use of more appropriately sized balloons for post-dilatation (3.3±0.37 vs. 3.19±0.44 mm, p=0.061). Procedural and fluoroscopy times were significantly longer in patients with calcific lesions (Table 1). Total BVS stent length was significantly higher in patients in the calcific group (52.1±26.2 vs. 43.8±25.3 mm, p=0.046).
PROCEDURAL OUTCOMES
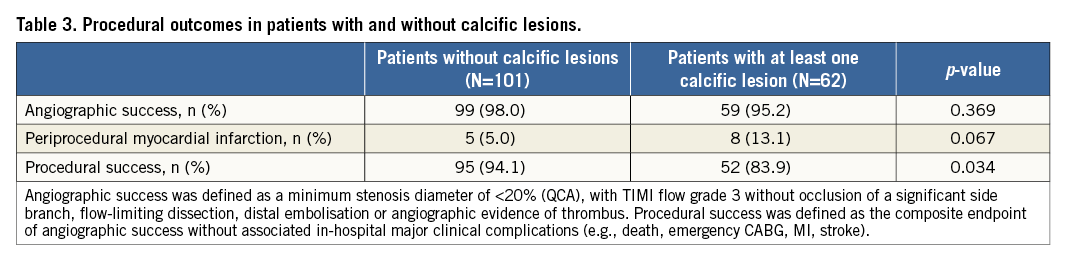
Post BVS implantation, a similar acute gain was obtained between calcific and non-calcific lesions (1.86±0.63 vs. 1.83±0.60 mm, p=0.732). Furthermore, at final IVUS, minimal scaffold area (mm2) was similar (calcific vs. non-calcific lesions; 6.23±1.78 vs. 6.09±2.34 mm2, p=0.672), and both groups achieved similar minimal lumen area gains (calcific vs. non-calcific lesions; 3.20±1.77 vs. 3.22±1.53 mm2, p=0.947). Even though angiographic success was similar (calcific vs. non-calcific 95.2% vs. 98%, p=0.369), there was a trend for a higher rate of periprocedural MI in the calcific group (13.1% vs. 5.0%, p=0.067), resulting in a significantly lower rate of procedural success (83.9% vs. 94.1%, p=0.034) (Table 3).
OUTCOMES IN PATIENTS WITH AND WITHOUT CALCIFIC LESIONS
Median follow-up time was 14 months (IQR 9.0 to 18.4 months). During the median follow-up time, 11 (10.9%) MACE were observed in the non-calcific group vs. eight (12.9%) in the calcific group (plog-rank=0.546) (Figure 1). There was only one death in the non-calcific group (plog-rank=0.428). No significant differences were observed in follow-up MIs (three [3.0%] in the non-calcific group vs. one [1.6%] in the calcific group, plog-rank=0.415). TLR occurred in eight (7.9%) patients with non-calcific lesions and in six (9.7%) patients in the calcific group (plog-rank=0.797) (Figure 1). There was one case of acute definite thrombosis in the non-calcific group and one case of late definite thrombosis in the calcific group (estimated one-year stent thrombosis rates: 1% vs. 1.9%, non-calcific vs. calcific, respectively, plog-rank=0.727).
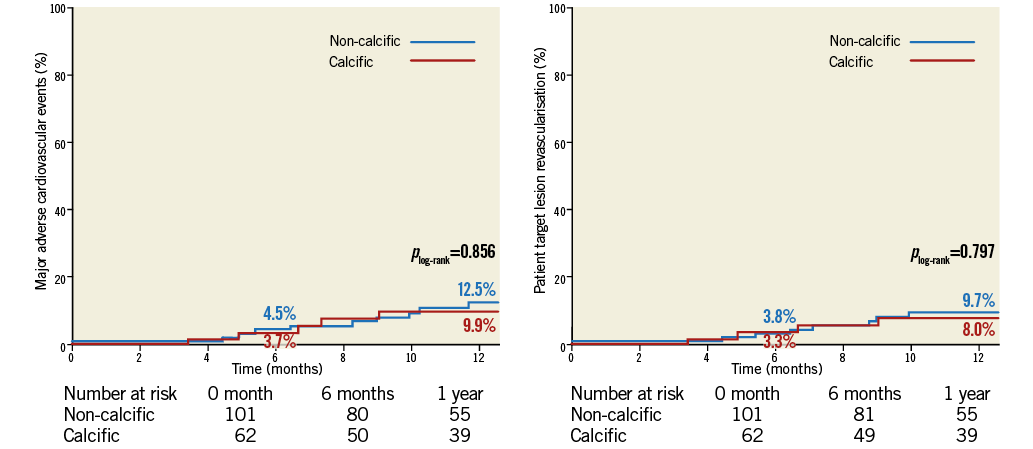
Figure 1. Kaplan-Meier curves suggesting no significant difference in one-year major adverse cardiovascular event and target lesion revascularisation rates in patients with and without calcific lesions treated with bioresorbable vascular scaffolds.
Discussion
The current study suggests that BVS implantation in calcified lesions is feasible, albeit at the cost of longer procedures and increased rates of periprocedural MI. Furthermore, it demonstrated acceptable short-term (one-year) MACE rates after BVS implantation in patients with calcific lesions. This comes at the expense of increased procedural and fluoroscopy times, increased use of IVUS, scoring balloons and rotablation, all of which facilitate meticulous lesion preparation and optimal stent apposition and expansion (Figure 2, Figure 3).
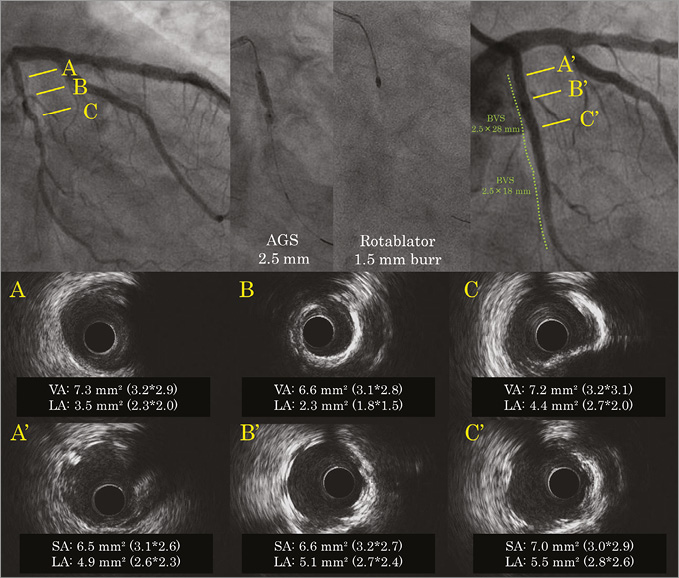
Figure 2. Angiographic and intravascular ultrasound (IVUS) images before (A, B, C) and after (A’, B’, C’) treatment of a left circumflex calcific lesion with bioresorbable vascular scaffolds. Baseline IVUS revealed a tight lesion with 180° of calcification and a minimum lumen area (MLA) of 2.3 mm2 (B) in the proximal left circumflex (LCx). The scoring balloon was unable to dilate the lesion, hence rotablation with a 1.5 mm burr was used. Final IVUS demonstrated a well-expanded scaffold with a lumen area of 5.1 mm2 and scaffold area of 6.6 mm2 at the MLA site (B’). AGS: AngioSculpt scoring balloon (AngioScore, Inc., Fremont, CA, USA); BVS: bioresorbable vascular scaffold; SA: scaffold area; VA: vessel area
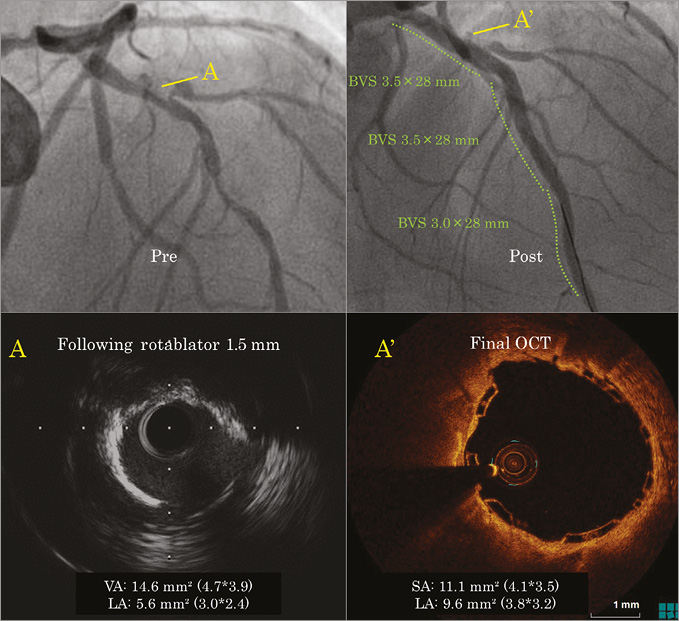
Figure 3. Bioresorbable vascular scaffold implantation in a proximal left anterior descending lesion demonstrating extensive circumferential calcification. Angiographic image before and after rotablation (top row). Bottom row: intravascular imaging following rotablation with a 1.5 mm burr (A). Optical coherence tomography image at the end of the procedure (A’) demonstrating the good result of a well-expanded scaffold with a lumen area of 9.6 mm2. BVS: bioresorbable vascular scaffold; OCT: optical coherence tomography; SA: scaffold area; VA: vessel area
BVS are considered by many to be the fourth revolution in coronary intervention20. The temporary scaffold, which prevents acute recoil and neointimal hyperplasia in the initial phase after implantation, gradually disappears, liberating the vessel from the negative effects of a metallic cage3,21,22. This promising technology aims to eliminate the fear of late stent thrombosis and help in the restoration of physiological vasomotion, leading to late luminal gain and late expansive remodelling. The future absorption of BVS allows future grafting of stented segments, facilitates reopening of “jailed” side branches23 and disappearance of overhang at ostial lesions. These benefits are of particular importance in patients with calcific lesions, who often have extensive coronary artery disease requiring long segments of stents (full metal jackets). Future studies are needed to demonstrate whether there is partial restoration of physiological vessel motion and expansive remodelling in non-circumferential calcific lesions or in lesions with circumferential calcium treated with atherectomy, cutting or scoring balloons.
Despite the plethora of new-generation stents and adjunctive technologies, calcific lesions remain a challenge for the interventional cardiologist. In a subanalysis of the randomised controlled TAXUS IV trial24 (1,314 patients randomised to bare metal stent [BMS] vs. paclitaxel-eluting stent [PES]), Moussa et al demonstrated a significant reduction in late lumen loss in calcific lesions (n=247) treated with PES vs. BMS (0.26±0.56 vs. 0.51±0.48 mm, p=0.015). In a study from the SPIRIT II trial25, the efficacy of EES in patients with at least one moderate calcific lesion (68 patients), determined angiographically, was compared to those without any calcific lesion (144 patients). Late lumen loss was similar between the two groups at two years (0.40±0.47 mm in the calcified group vs. 0.31±0.28 mm in the non-calcified group, p=0.54). No significant difference in two-year MACE rates was observed between the two groups (calcific vs. non-calcific: 10.9% vs. 4.4%, p=0.12). The numerically increased MACE rate was attributed to an increased ischaemia-driven TLR (7.8% vs. 1.5%, p=0.03). In a pooled analysis of seven first- and second-generation DES trials9, Bourantas et al revealed severe coronary calcification (assessed by angiography) to be an independent marker of three-year all-cause mortality (HR 1.33, 95% CI: 1.00 to 1.77, p=0.047) and the composite outcome of death, MI and any revascularisation (HR 1.18, 95% CI: 1.01 to 1.39, p=0.042). These worse outcomes in patients with calcific lesions may be partially attributed to suboptimal or incomplete revascularisation reflected in higher residual SYNTAX scores, a powerful determinant of prognosis26.
Treating lesions in non-compliant vessels increases the odds of stent underexpansion, and difficulties in device delivery may strip the polymeric material with ensuing compromise in local drug elution. Stent underexpansion may be subtle angiographically and overlooked in many cases. Hence, IVUS use and QCA are of utmost importance when treating calcific lesions. According to proposed strict criteria by de Jaegere et al27, excellent expansion is evident when the minimum lumen area in the stent is ≥90% of the average reference lumen area. In severely calcific long lesions, extensive decalcification using rotational or orbital28 atherectomy followed by extensive DES implantation appears less attractive due to increased rates of future TLR11. However, the advent of BVS may justify a decalcification strategy followed by bioresorbable vascular scaffold implantation for diffusely diseased, calcific arteries29. However, unlike the second- and third-generation DES, the delivery of first-generation BVS in calcified lesions can be more challenging due to their strut thickness (Absorb: 157×191/210 µm)30. Furthermore, when stretched beyond its designed limits and after partial bioabsorption as time goes by, a BVS may lose some of its radial strength and may be prone to acute or late recoil.
The findings of the current study alleviate some of these concerns, by demonstrating that BVS implantation is feasible in calcific lesions with acute gain similar to that of non-calcific lesions, albeit at the expense of increased procedural times, increased use of dedicated devices and increased rates of periprocedural MI. One-year MACE rates appear acceptable in patients with and without calcific lesions.
Limitations
Limitations of the current study include the small sample size, the relatively short follow-up and the lack of routine angiographic and intracoronary imaging follow-up. Furthermore, operators used IVUS predominantly for complex lesions, hence this selection bias may have influenced procedural and intermediate-term outcomes.
Conclusions
In conclusion, this is the first study to demonstrate the feasibility of BVS implantation in calcific lesions with acceptable periprocedural and one-year outcomes. Meticulous lesion preparation and the use of dedicated devices, such as scoring balloons and rotablation, facilitate optimal stent expansion and apposition at the expense of increased procedural and fluoroscopy times and higher rates of periprocedural MI. Future large studies with long-term follow-up, including routine angiographic and intravascular imaging follow-up, are eagerly awaited to identify the role of BVS in the treatment of calcific lesions.
| Impact on daily practice The current study suggests that implantation of bioresorbable vascular scaffolds in calcific lesions is feasible, with acceptable periprocedural and one-year outcomes. Operators should pay particular attention to meticulous lesion preparation with dedicated devices when needed, in order to achieve optimal stent apposition and expansion. |
Conflict of interest statement
The authors have no conflicts of interest to declare.

