
Based on the instructions for use, the current version of the Absorb bioresorbable scaffold (Absorb GT1; Abbott Vascular, Santa Clara, CA, USA) should not be used to treat severely calcified lesions which have not had “adequate lesion preparation”. As such, the manufacturer does not contraindicate using the device in calcified lesions, but warns against its usage in heavily calcified lesions which have not been properly dilated. Indeed, severe coronary calcification is a known independent predictor of poorer prognosis in patients undergoing percutaneous coronary intervention (PCI) with drug-eluting stents (DES)1. In addition to creating delivery issues and the potential to damage the device, the geographical extent of lesion calcification is associated with underexpansion2, and underexpansion is a contributory factor to late failure, not only in the DES, but also in the scaffold era3.
The study protocol of ABSORB III, a pivotal trial of the Absorb scaffold versus second-generation DES, excluded patients with moderate or heavy calcification proximal to or within the target lesion4. If intravascular ultrasound (IVUS) was used, subjects were excluded if the calcium arc in the vessel prior to the lesion or within the lesion was ≥180°. As such, the ABSORB III study does not inform on the outcome of moderately or severely calcified lesions treated with the Absorb device, and might not reflect a reliable snapshot of extended use of coronary scaffolds in pioneering centres worldwide. While the positive effect of scaffolds on vasomotion may not materialise in calcified lesions, many interventionalists would agree that effects such as conformability, late lumen enlargement, and disappearance of late uncovered struts and inflammatory processes might still apply in this subset5.
In this issue of the Journal, Panoulas et al provide novel insights into the topic, reporting on a total of 163 patients who received the Absorb scaffold at two centres in Milan, Italy, over a two-year period6. Lesions were considered calcified if they presented a >90° arc of calcium by IVUS, or if they presented with moderate or severe calcification by angiography (i.e., in the absence of IVUS). Based on this definition, patients with at least one calcified lesion (n=62) were more likely to be diabetic (35.5% vs. 22.8%) and with chronic kidney disease (31.1% vs. 13.9%) compared with those without calcified lesions (n=101). Not surprisingly, there was a more extensive use of aggressive plaque modification strategies in the calcified lesions group, including predilatation in 88%, scoring in 26% and rotational atherectomy in 12%. All lesions were post-dilated, but larger non-compliant balloons were used for post-dilatation in patients with at least one calcified lesion. Reassuringly, there were no differences in post-implant quantitative coronary angiography or IVUS metrics between calcified and non-calcified lesions, including comparable scaffold and lumen areas. On average, these outcomes were achieved at the price of 17 more ml of contrast medium administration, eight more minutes of fluoroscopy time and 32 more mGycm2 of radiation dose in patients with calcified lesions. Although the rates of angiographic success were similar (95.2% vs. 98% in patients with at least one calcified lesion vs. those without, respectively; p=0.369), procedural success was significantly lower in the calcific group (83.9% vs. 94.1%, p=0.034), due to a higher incidence of periprocedural myocardial infarction trending towards statistical significance (13.1% vs. 5.0%; p=0.067). The authors reported no differences in the rate of major adverse cardiac events at 12 months, but the comparison of clinical endpoints was clearly underpowered.
A number of issues should be taken into account when appraising the results of the study from Panoulas et al. First, to test their hypothesis of the Absorb scaffold performing acceptably in calcified lesions, the authors selected a control group of patients who also received the device but did not present with calcified lesions. As such, we are not given information on the benefit-risk profile of the Absorb scaffold for calcified lesions compared with the current standard of care (i.e., second-generation DES). Second, IVUS (available in 78% of cases) was used in almost all patients with at least one calcified lesion versus about two thirds of patients without. This kind of ascertainment bias may have resulted in patients with calcified lesions not actually being identified as such. Indeed, relying on angiography for detecting calcification reflects common practice but has known limitations7. IVUS itself has a limitation, in that only the arc and length of calcium can be measured, but not the mass or axial depth. Third, the mean calcium arc by IVUS in the study was 183°, which largely corresponds to moderate calcification by visual angiographic estimation. Based on their chosen cut-off for defining calcification, the investigators also potentially included patients with a calcium arc just above 90°, which may correspond to mild calcification angiographically7. In this regard, a breakdown of the results by degree of calcification would have been of interest. Also of interest would have been to appreciate how IVUS affected the operator’s decision making after scaffold implant in the two groups. Other obvious and understandable limitations pertain to the small number of patients included, the availability of a relatively short follow-up, the absence of angiographic follow-up, and the lack of statistical adjustment for residual confounding that prevent meaningful comparisons on clinical grounds. With regard to the latter, it should be noted that some baseline imbalances acted against the calcified lesions group (i.e., diabetes, chronic kidney disease), and therefore the finding of similar event rates at 12 months is reassuring. Finally, patients were treated in two high-volume Italian centres by operators with a great experience with calcified lesions in general and the Absorb scaffold in particular, which may have impacted on generalisability. Even so, it should be noted that the number of calcific lesions treated with scaffolds in two years by each of these centres was only about 1.3 per month.
Overall, in an unexplored scenario, Panoulas et al have now demonstrated that placing scaffolds in adequately prepared calcified lesions is feasible, with no apparent detrimental effect on acute lumen gain and scaffold areas compared with non-calcified lesions. This should not be considered a “free lunch” even by the most enthusiastic scaffold implanters, due to the increased procedural times, use of dedicated devices and the higher rates of periprocedural myocardial infarction (this latter being a debated outcome). One may argue that more time and resources are spent even when DES are used for calcified compared with non-calcified lesions, but scaffolds require longer procedures than DES in non-complex lesions4, so this difference will probably be amplified in head-to-head comparisons if the technical coefficient increases. On top of other considerations, we are now realising that “operator motivation” is one of the most important criteria when dealing with selection of stents or scaffolds for challenging or “off-label” anatomies. Calcified lesions can be treated with scaffolds if one is ready eventually to embark on using dedicated balloons or atherectomy for mechanical debulking (Figure 1)5. Surely, patients with unfortunate angiographic features deserve our time and dedication, including extensive use of resources as required to get the best possible procedural outcome. But are coronary scaffolds worth this effort? This is a question for the long-term follow-up of ongoing large-scale trials, and future multicentre registries including broader lesions than those represented therein.
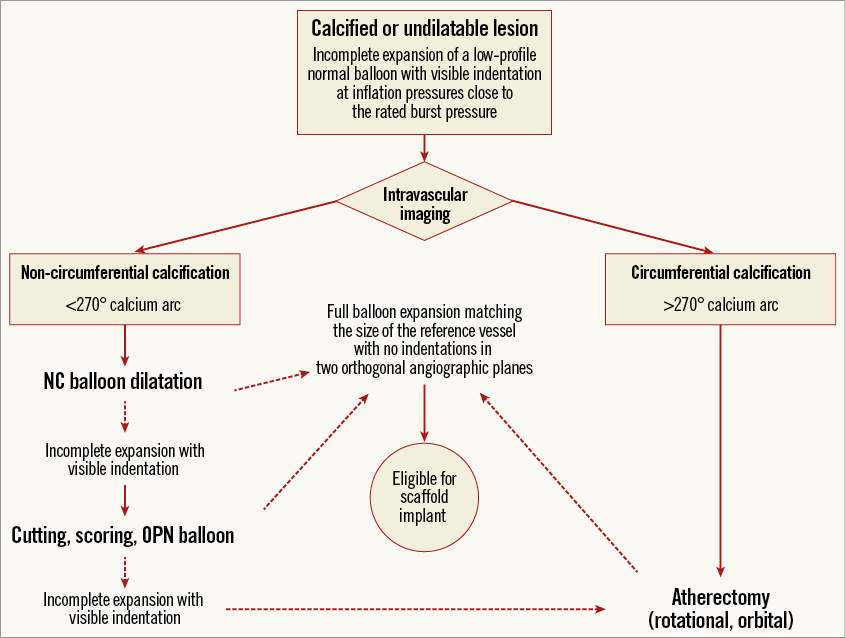
Figure 1. Proposed stepwise algorithm for lesion preparation before scaffold placement in calcified lesions.
Conflict of interest statement
The author has received speaker’s honoraria from Abbott Vascular.

