
Abstract
Aims: We aimed to investigate the impact of lesion calcification on angiographic outcomes after Absorb everolimus-eluting bioresorbable vascular scaffold (BVS) implantation in comparison with those after cobalt-chromium everolimus-eluting stent (CoCr-EES) implantation.
Methods and results: The present post hoc analysis of the ABSORB Japan randomised trial compared post-procedure and 13-month angiographic outcomes between patients implanted with BVS and CoCr-EES based on the presence or absence of calcification, excluding extremely heavily calcified lesions or lesions requiring rotational atherectomy. The study population comprised 384 patients with 384 lesions (including 114 lesions [29.7%] with moderate or severe calcification), classified into two subgroups: calcification, 114 (BVS: n=72 and CoCr-EES: n=42) and non-calcification, 270 (BVS: n=181 and CoCr-EES: n=89). Follow-up angiography was performed in 94.8% of patients. Both post-procedure and follow-up in-device minimal lumen diameters were comparable in both the BVS arm (calcification vs. non-calcification: 2.43±0.32 mm vs. 2.43±0.39 mm, p=0.91 and 2.17±0.49 mm vs. 2.27±0.47 mm, p=0.17) and in the CoCr-EES arm (2.68±0.34 mm vs. 2.65±0.42 mm, p=0.62 and 2.57±0.52 mm vs. 2.47±0.53 mm, p=0.36).
Conclusions: Moderate or severe lesion calcification (excluding patients with extremely heavily calcified lesions or lesions requiring rotational atherectomy) does not negatively affect angiographic outcomes at both post-procedure and 13-month follow-up after BVS implantation. ClinicalTrials.gov Identifier: NCT01844284
Abbreviations
BVS: bioresorbable vascular scaffold
CoCr-EES: cobalt-chromium everolimus-eluting stent
DES: drug-eluting stent
MLD: minimal lumen diameter
OCT: optical coherence tomography
Introduction
Drug-eluting stents (DES) have reduced the rates of restenosis and subsequent target lesion revascularisation compared with bare metal stents1; however, long-term safety concerns, such as stent thrombosis and late restenosis, remain due to the permanent presence of polymer and metallic prostheses within the vessel2, for which reason the bioresorbable vascular scaffold (BVS), a drug-eluting fully bioresorbable semicrystalline poly-L-lactide scaffold, has been developed. However, BVS deployment in complex lesions is challenging due to the intrinsic character of the poly-L-lactide backbone, and lesion calcification can cause inadequate expansion and recoil of BVS3, which are prone to scaffold thrombosis and restenosis4. Therefore, we investigated the impact of lesion calcification on angiographic and clinical outcomes after BVS implantation in comparison with DES implantation based on the data from the ABSORB Japan trial.
Methods
STUDY POPULATION AND PROTOCOL
The ABSORB Japan trial was a prospective, multicentre, randomised, single-blind, active-controlled clinical trial of 400 patients undergoing percutaneous coronary intervention. The patients received either the Absorb™ everolimus-eluting BVS (Abbott Vascular, Santa Clara, CA, USA) or a cobalt-chromium everolimus-eluting stent (CoCr-EES) (XIENCE PRIME®/ Xpedition®; Abbott Vascular) on a 2:1 basis. Details of the organisational structure, the 38 participating centres, the study design, and the clinical and angiographic outcomes at one year have been described previously5. Our study is a post hoc analysis of the ABSORB Japan trial to investigate the impact of lesion calcification on post-procedure and 13-month angiographic outcomes following BVS versus CoCr-EES implantation. Patients were classified into two subgroups according to the presence or absence of calcification: moderate or severe calcification (calcification group) and no or mild calcification (non-calcification group). The study protocol excluded patients with extremely heavy lesion calcification found by on-site investigators. In our study, patients with multiple target lesions were excluded in order to avoid the mixture of calcified and non-calcified lesions in one patient, although the study protocol allowed treatment of up to two target lesions. In addition, one patient who had received inadequate assessment of lesion calcification was excluded. Finally, our study population consisted of 384 patients with 384 lesions (BVS, n=253 and CoCr-EES, n=131) (Figure 1).
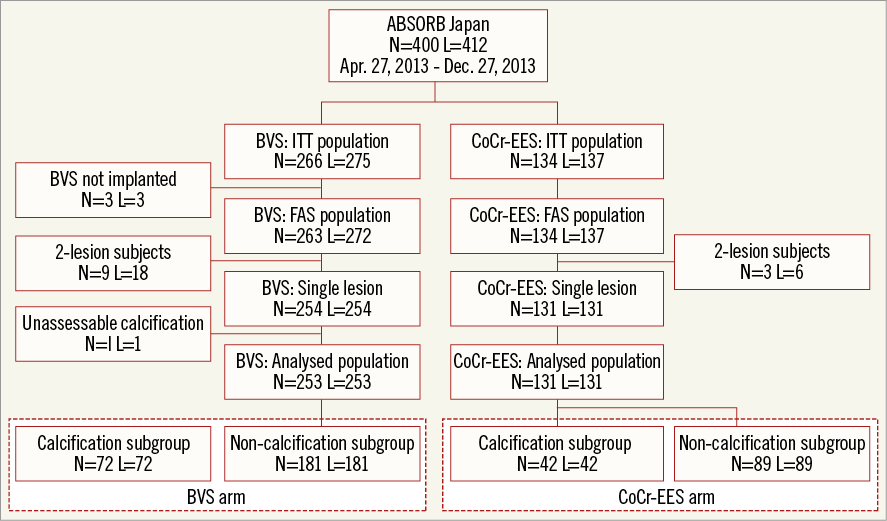
Figure 1. Study flow chart. BVS: bioresorbable vascular scaffold; CoCr-EES: cobalt-chromium everolimus-eluting stent; FAS: full-analysis set; ITT: intention-to-treat; L: number of lesions; N: number of patients
The study was carried out in accordance with the provisions of the Declaration of Helsinki and the guidelines for epidemiological studies issued by the Ministry of Health, Labor, and Welfare of Japan. The clinical trial protocol was approved by each institutional review board. All patients provided written informed consent before enrolment.
ENDPOINTS
The primary outcome measures were angiographic in-device minimal lumen diameter (MLD) at post-procedure and 13-month follow-up. An independent angiographic analysis of all baseline and follow-up angiograms was performed using QAngio® XA 7.3 (Medis medical imaging systems bv, Leiden, The Netherlands) at an angiographic core laboratory (Beth Israel Deaconess Medical Center Angiographic Core Laboratory, Boston, MA, USA). Calcification was defined as apparent density noted within the vascular wall at the stenotic site. The severity of calcification was classified as none or mild, moderate (density seen only with cardiac motion before contrast medium injection on one side of the arterial wall), and severe (radiopacity seen without cardiac motion before contrast medium injection generally on both sides of the arterial wall)6. The final adjudication of the severity of calcification was made at the angiographic core laboratory. An independent analysis of post-procedural optical coherence tomography (OCT) findings was performed at an OCT core laboratory (Cardialysis BV, Rotterdam, The Netherlands), except for the measurement of the embedment of stent/scaffold struts, which was performed by an academic team in Rotterdam with QCU-CMS software version 4.69 (Leiden University Medical Center, Leiden, The Netherlands)7.
We also investigated the clinical outcomes at 12 months as an exploratory analysis. Target lesion failure was defined as a composite of cardiac death, myocardial infarction attributable to the target vessel, and ischaemia-driven target lesion revascularisation at 12 months. Target vessel failure was defined as a composite of cardiac death, myocardial infarction, and ischaemia-driven target vessel revascularisation. Periprocedural myocardial infarction was defined as elevation of the creatinine kinase-MB level greater than five times the upper limit of normal, and spontaneous myocardial infarction as that greater than the upper limit of normal. Definite stent or scaffold thrombosis was defined according to the Academic Research Consortium definitions8. Target lesion revascularisation was defined as any repeat percutaneous coronary intervention or coronary artery bypass surgery.
STATISTICAL ANALYSIS
For binary variables, counts and percentages were calculated, and the p-value from Pearson’s chi-square test was used when Cochran’s rule was met. Otherwise, Fisher’s exact test was used. For continuous variables, means, standard deviations, and t-tests were performed when appropriate. P-values less than 0.05 were considered statistically significant. All statistical analyses were performed using SAS 9.2 and 9.3 (SAS Institute Inc., Cary, NC, USA).
Results
STUDY POPULATION
Moderate or severe calcification was present in 28.5% (72/253 lesions) of the BVS arm and 32.1% (42/131 lesions) of the CoCr-EES arm. Accordingly, the calcification subgroup comprised 114 lesions (BVS, n=72 and CoCr-EES, n=42, including moderate calcification, n=82 and severe calcification, n=32), and the non-calcification subgroup comprised 270 lesions (BVS, n=181 and CoCr-EES, n=89) (Figure 1).
BVS VERSUS CoCr-EES IN THE CALCIFICATION AND NON-CALCIFICATION SUBGROUPS
Baseline clinical and lesion characteristics were comparable between BVS and CoCr-EES in both subgroups, except for a significantly higher prevalence of unstable angina in CoCr-EES of the non-calcification subgroup (Table 1). Procedural characteristics were comparable between BVS and CoCr-EES in both subgroups, with high device and procedure success rates (Table 2).
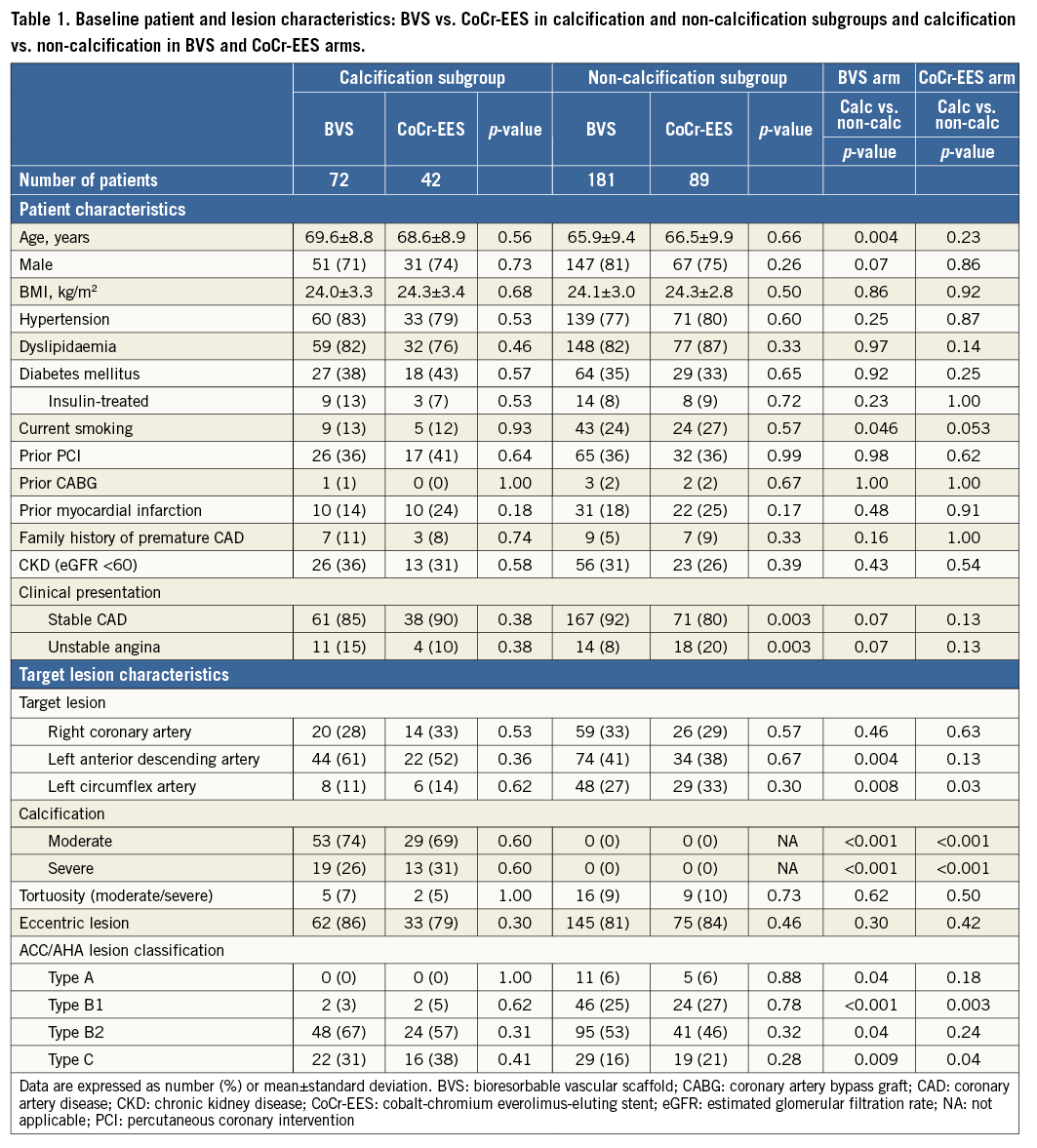
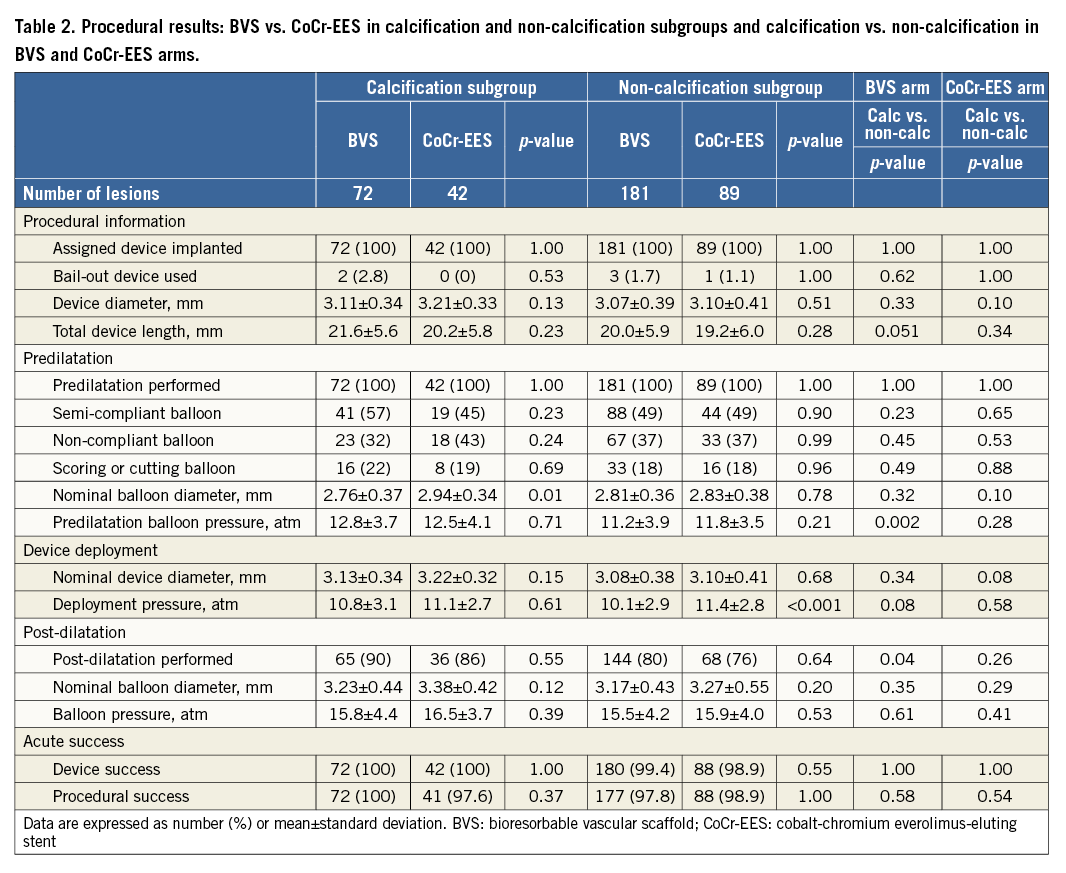
At baseline, quantitative angiographic parameters were comparable between BVS and CoCr-EES in both subgroups, except for a significant difference in MLD in the calcification subgroup (Table 3). At post procedure, in-device MLD was significantly smaller in BVS in both subgroups, while in-segment MLD was not significantly different (Table 3, Figure 2). Post-procedural incomplete stent/scaffold apposition measured on OCT imaging trended to be less prevalent in the BVS arm than in the CoCr-EES arm, regardless of the presence of calcification. The embedment depth of the struts was greater in the CoCr-EES arm than in the BVS arm (Table 4).
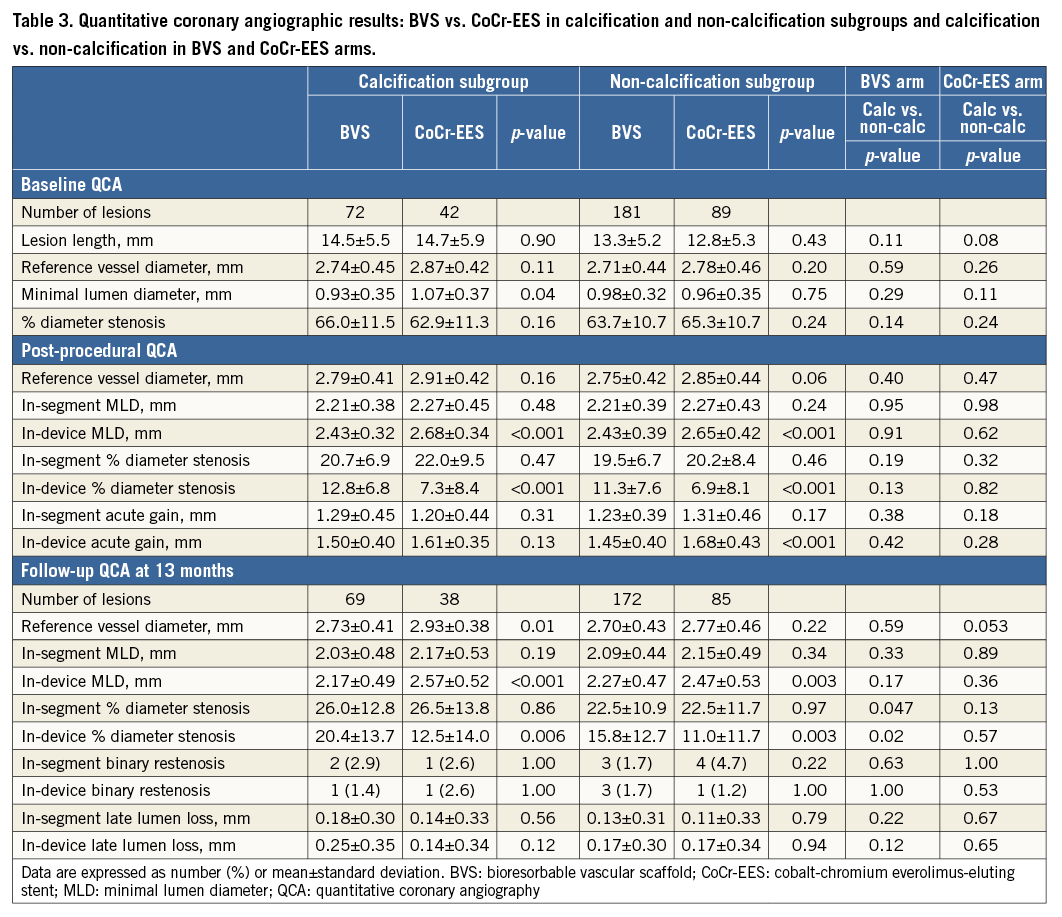
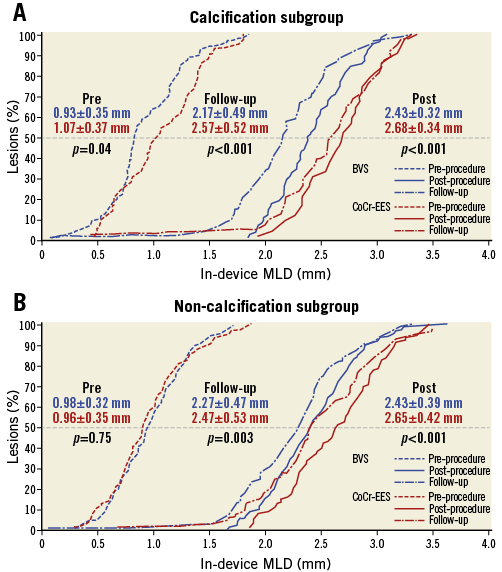
Figure 2. Cumulative distribution function curves for in-device MLD: BVS vs. CoCr-EES. A) Calcification subgroup. B) Non-calcification subgroup. BVS: bioresorbable vascular scaffold; CoCr-EES: cobalt-chromium everolimus-eluting stent; MLD: minimal lumen diameter
Thirteen-month follow-up coronary angiography was performed in 94.8% of patients (364/384). In-device MLD was significantly smaller in BVS in both subgroups, while in-segment MLD was not significantly different. Both in-device and in-segment late lumen loss was comparable between BVS and CoCr-EES in both subgroups (Table 3, Figure 2).
Clinical outcomes were comparable between BVS and CoCr-EES in both subgroups (Table 5). The rate of ischaemia-driven target lesion revascularisation at 12 months was similar between BVS and CoCr-EES in both calcification (2.8% vs. 2.4%) and non-calcification (2.8% vs. 2.3%) subgroups.
CALCIFICATION VERSUS NON-CALCIFICATION IN THE BVS AND CoCr-EES ARMS
Baseline patient and lesion characteristics (Table 1) showed that patients with calcified lesions were older, smoked less, and had more left anterior descending target lesions regardless of the treatment assignment. The procedural results were comparable between patients with and without calcified lesions, except for the significantly higher predilatation balloon pressure and rate of post-dilatation in patients with calcified lesions in the BVS arm, whereas no significant difference existed in the CoCr-EES arm (Table 2).
Quantitative angiographic results were similar between patients with and without calcified lesions in both treatment arms. In-device and in-segment MLD at post-procedure and 13-month follow-up was also comparable in both treatment arms (Table 3). Cumulative distribution curves of in-device MLD are shown in Figure 3. The degree of post-procedural incomplete stent/scaffold apposition and the embedment depth of struts measured on OCT imaging were not significantly different between the calcification and non-calcification subgroups irrespective of the device type (Table 4).
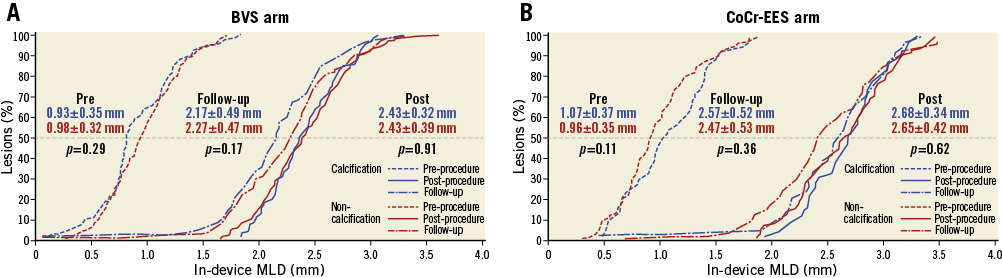
Figure 3. Cumulative distribution function curves for in-device MLD: calcification vs. non-calcification. A) BVS arm. B) CoCr-EES arm. BVS: bioresorbable vascular scaffold; CoCr-EES: cobalt-chromium everolimus-eluting stent; MLD: minimal lumen diameter
Clinical outcomes were comparable between patients with and without calcified lesions in both treatment arms (Table 5). A very low 12-month rate of ischaemia-driven target lesion revascularisation was observed in both BVS (calcification vs. non-calcification: 2.8% vs. 2.8%) and CoCr-EES (2.4% vs. 2.3%) arms.
Discussion
Our primary findings were as follows: 1) the device and procedure success rates of BVS implantation were comparable to those of CoCr-EES implantation regardless of the presence or absence of calcification; 2) in both calcified and non-calcified lesions, in-device MLD at post-procedure and 13-month follow-up was significantly smaller in the BVS arm than in the CoCr-EES arm, while in-segment MLD was not significantly different; and 3) both in-device and in-segment MLD at post-procedure and 13-month follow-up was comparable regardless of the presence or absence of calcification in both treatment arms.
In the ABSORB Japan trial, nearly 30% of the patients had lesions with moderate or severe calcification, suggesting that moderately calcified lesions can be detected in daily practice. The long-term outcomes after DES implantation were reported to be worse in patients with calcified lesions than in those without calcified lesions9-11. Our comparable angiographic outcomes following BVS implantation confirm the use of BVS in calcified lesions. However, meticulous attention should be paid to the technical issues to achieve optimal scaffold expansion in calcified lesions because the predilatation balloon pressure and rate of post-dilatation in the BVS arm of our study were higher in calcified lesions. On the other hand, the use rate of scoring or cutting balloons for predilatation was nearly the same irrespective of the presence or absence of calcification. However, it did not have a detrimental effect on the 13-month angiographic or 12-month clinical outcomes12. The ABSORB Japan trial included focal lesions which were typical for the pivotal trials of DES implantation. Therefore, our findings should not be extended to long or diffuse lesions with moderate or severe calcification.
The presence of lesion calcification often hinders smooth scaffold delivery. Even if scaffold deployment is achieved, acute strut malapposition or suboptimal scaffold expansion can happen more often in calcified lesions13,14. A systematic review reported that lesion calcification was associated with the risk of stent thrombosis after DES implantation15. Malapposed struts and suboptimal scaffold expansion are prone to acute or subacute scaffold thrombosis16-18. Furthermore, acute strut malapposition of BVS can cause subsequent strut discontinuity during the advanced resorption period, which predisposes to very late scaffold thrombosis19. Adequate lesion preparation and high-pressure post-dilatation with an appropriately sized balloon are important to avoid acute strut malapposition and to achieve optimal scaffold expansion, although the expansion limit of the current BVS might hinder full strut apposition20.
Study limitations
This is a post hoc analysis of the ABSORB Japan trial without any adequate power to compare the clinical outcomes as well as angiographic outcomes between the calcified and non-calcified lesions. The clinical outcomes from a relatively small study population need careful interpretation. Moreover, we excluded patients with multiple target lesions because a patient could have both calcified and non-calcified lesions, which confuses the assessment of patient characteristics according to the presence or absence of calcification; however, the number of patients excluded was very small. Our BVS procedural characteristics, such as the use of relatively small-sized balloons for predilatation and the low use rate of non-compliant, scoring or cutting balloons for calcified lesions, were technically distinct from the current expert recommendations in Europe21. Our procedural characteristics might have influenced the findings in this study. The angiographic evaluation of lesion calcification might have limited sensitivity to detect small amounts of calcification6,22. In addition, the OCT follow-up data were not included because they only became available two years after the procedure. Finally, patients with extremely heavily calcified lesions or lesions requiring rotational atherectomy were excluded. Consequently, our results cannot be applied to the extremely heavily calcified lesions excluded by the protocol.
Conclusions
Moderate or severe lesion calcification, excluding patients with extremely heavily calcified lesions or lesions requiring rotational atherectomy, does not negatively affect angiographic outcomes at both post-procedure and 13-month follow-up after BVS implantation.
| Impact on daily practice The present post hoc analysis of the ABSORB Japan trial suggests that moderate or severe lesion calcification, excluding patients with extremely heavily calcified lesions or lesions requiring rotational atherectomy, does not negatively affect angiographic outcomes at both post-procedure and 13-month follow-up after BVS implantation. When treating calcified lesions with BVS, however, meticulous attention should be paid to the technical issues to achieve optimal scaffold expansion. |
Guest Editor
This paper was guest edited by Antonio Colombo, MD, FACC, FESC, FSCAI; Department of Interventional Cardiology, EMO GVM Centro Cuore Columbus, Milan, Italy.
Funding
This study was funded by Abbott Vascular. The sponsor was involved in the study design, data collection, analysis, and interpretation.
Conflict of interest statement
T. Kimura, J. Popma, and P. Serruys are members of the Advisory Board of Abbott Vascular. T. Kimura, K. Kozuma, K. Tanabe, and Y. Onuma are members of the Advisory Board of Abbott Vascular Japan. S. Ying and H. Kusano are employees of Abbott Vascular. G. Stone is a consultant to Reva Corp. The other authors have no conflicts of interest to declare. The Guest Editor has no conflicts of interest to declare.

