
Abstract
Aims: Absorb bioresorbable vascular scaffolds (BVS) and XIENCE cobalt-chromium everolimus-eluting stents (CoCr-EES) had comparable angiographic and clinical outcomes up to one year in patients enrolled in the ABSORB China randomised trial. Whether these favourable results with BVS continue beyond one year up to three years is unknown. In this study we sought to analyse the outcomes from the trial up to three-year follow-up..
Methods and results: ABSORB China was a prospective, open-label, multicentre trial in which 480 patients with one or two native coronary artery lesions were randomised 1:1 to BVS (N=241) vs. CoCr-EES (N=239). Clinical endpoints included target lesion failure (TLF; cardiac death, target vessel-related myocardial infarction or ischaemia-driven target lesion revascularisation), its components, and definite/probable stent/scaffold thrombosis (ST). There were no significant differences in clinical outcomes in patients treated with BVS and CoCr-EES up to three years, including TLF (5.5% vs. 4.7%, p=0.68) and definite/probable ST (0.9% vs. 0.0%, p=0.50). STs in the BVS arm consisted of one probable subacute event at 15 days and one definite very late event at 622 days. Among 32 BVS patients with a reference vessel diameter between 2.25 and 3.75 mm by quantitative coronary angiography and in whom post-dilatation was performed at >16 atm with a balloon:scaffold diameter >1:1 and balloon ≤scaffold diameter 0.5 mm, no TLF or ST events occurred within three years.
Conclusions: In the ABSORB China trial, BVS and CoCr-EES had similar results up to three-year follow-up, the time at which the scaffold has completely resorbed. BVS outcomes may be further optimised by appropriate lesion selection and implantation technique. ClinicalTrials.gov identifier: NCT01923740. https://clinicaltrials.gov/ct2/show/NCT01923740
Abbreviations
ARC: Academic Research Consortium
BVS: bioresorbable vascular scaffold
CK: creatine kinase
CoCr-EES: cobalt-chromium everolimus-eluting stent
DAPT: dual antiplatelet therapy
DoCE: device-oriented composite endpoint
ID-TLR: ischaemia-driven target lesion revascularisation
ID-TVR: ischaemia-driven target vessel revascularisation
ITT: intent-to-treat
KM: Kaplan-Meier
MACE: major adverse cardiac events
MLD: minimum lumen diameter
NQMI: non-Q-wave myocardial infarction
PCI: percutaneous coronary intervention
PoCE: patient-oriented composite endpoint
PSP: predilatation, vessel sizing, and post-dilatation
QCA: quantitative coronary angiography
RVD: reference vessel diameter
ST: scaffold/stent thrombosis
TV-MI: target vessel-related myocardial infarction
TLF: target lesion failure
TVF: target vessel failure
Introduction
Bioresorbable vascular scaffolds (BVS) represent a potential breakthrough advance in percutaneous coronary intervention (PCI), as the absence of a permanent metallic cage deforming and limiting vascular responses in the coronary tree may provide long-term clinical benefits (a hypothesis currently being tested in large-scale randomised trials)1. The safety and effectiveness of the everolimus-eluting poly-L-lactic acid-based Absorb™ BVS (Abbott Vascular, Santa Clara, CA, USA) has been extensively tested, and seven randomised controlled trials have demonstrated non-inferior one-year results with BVS compared to XIENCE® cobalt-chromium everolimus-eluting stents (CoCr-EES; Abbott Vascular)2-8. In an individual patient-level pooled meta-analysis of 3,389 patients from the ABSORB II, ABSORB III, ABSORB China and ABSORB Japan trials, comparable results between BVS and CoCr-EES were reported for one-year patient-oriented and device-oriented outcomes, although an increased risk of target vessel-related myocardial infarction (TV-MI) was present with BVS, attributed in part to a non-significant trend towards increased scaffold thrombosis (ST)9. Early registry studies and study-level meta-analyses have also suggested that BVS was associated with an increased risk of ST within one year10-12. Fewer data are available for the long-term outcomes of BVS. Late results from the single-arm ABSORB EXTEND and ABSORB Cohort B studies showed a good safety profile of BVS at three and five years, respectively13. However, recent data from the randomised ABSORB Japan (two years), ABSORB III (two years), ABSORB II (three years), and AIDA (mean two-year follow-up) trials demonstrated a higher rate of very late stent/scaffold thrombosis with BVS compared to CoCr-EES8,14,15, which have raised concerns over the long-term outcomes of BVS during the active bioresorption process. The results after one year from the ABSORB China trial have not yet been published.
Furthermore, since the performance of the ABSORB randomised trials, the importance of vessel size and procedural technique to ensure optimal patient outcomes has become recognised. BVS has a strut thickness of 157 µm, and vessels with a reference vessel diameter (RVD) <2.25 mm by quantitative coronary angiography (QCA) have been found to have an increased rate of ST with BVS, reflecting especially high surface area coverage and polymer load in such vessels. In addition, deployment strategies specific for BVS, including high-pressure scaffold post-dilatation, have been associated with a reduction in the one-year ST rate by ~70%16. Whether application of these techniques within the framework of the completed randomised trials is associated with improved outcomes has not been formally examined.
We therefore analysed and herein report the outcomes from the ABSORB China randomised trial up to three years, with analysis of device outcomes according to optimal vessel sizing and technique considerations.
Methods
PROTOCOL AND PATIENT POPULATION
The study design and one-year results from the ABSORB China trial have previously been reported in detail4. Briefly, ABSORB China was a prospective, active-controlled, open-label, multicentre clinical trial in which 480 patients were randomised 1:1 to BVS vs. CoCr-EES at 24 sites in China. Up to two de novo native coronary artery lesions were randomised per patient, each in a different epicardial vessel. The target lesion(s) were required to have an RVD based on visual estimation or a maximum lumen diameter based on on-line QCA of ≥2.5 mm and ≤3.75 mm with lesion length ≤24 mm. Predilatation of the target lesion was mandatory, whereas post-dilatation was left to the discretion of the investigator. If post-dilatation of an implanted BVS was needed, the expanded diameter of the post-dilatation balloon was required to be no more than 0.5 mm larger than the nominal diameter of the scaffold to avoid acute scaffold fracture. Intravascular imaging was not encouraged for vessel sizing or procedural guidance.
The study was reviewed and approved by the ethics committee of each of the study sites. All patients signed informed consent prior to enrolment. Angiographic follow-up was planned in all patients at one year, and clinical follow-up was performed at 30, 180 and 270 days and at 1, 2, 3, 4 and 5 years. Follow-up is complete up to three years at the time of the present report. An ADP antagonist was continued for at least 12 months, and aspirin was administered for the five-year duration of the study. On-site monitoring of 100% of the trial data was performed. All angiograms were analysed by an independent angiographic core laboratory, and clinical endpoint events were adjudicated by an independent clinical events committee. The study was sponsored by Abbott Vascular, and is registered on ClinicalTrials.gov (NCT01923740).
ENDPOINTS AND DEFINITIONS
The primary endpoint was in-segment late loss at one year, powered to demonstrate non-inferiority of BVS to CoCr-EES. Key clinical secondary endpoints included the device-oriented composite endpoint (DoCE) of target lesion failure (TLF; cardiac death, TV-MI, or ischaemia-driven target lesion revascularisation [ID-TLR]), the patient-oriented composite endpoint (PoCE; all-cause death, all MI, or all revascularisation), target vessel failure (TVF; cardiac death, MI, or ischaemia-driven target vessel revascularisation [ID-TVR]), major adverse cardiac events (MACE; cardiac death, MI, or ID-TLR), the individual component endpoints of these endpoints, and ST. ST was defined based on timing of the event (acute [<24 hours], subacute [one to 30 days], late [30 days to one year] and very late [one to three years]) and level of evidence (definite or probable) according to the Academic Research Consortium (ARC) definitions17. Periprocedural non-Q-wave MI (NQMI) was defined as a rise in post-PCI creatine kinase-myocardial band (CK-MB) to >5 times the upper reference limit. Other endpoint definitions were standard, and have been described previously4.
TECHNIQUE ANALYSIS
An exploratory analysis was undertaken to assess the impact of procedure technique, termed PSP, including predilatation, vessel sizing, and post-dilatation, on the clinical outcomes of the BVS implantation. For the present study, optimal PSP was defined as being present if the following three conditions were met: 1) predilatation performed; 2) vessel properly sized (2.25 mm ≤RVD ≤3.5 mm by QCA); and 3) post-dilatation performed at high pressure (>16 atm) and with an appropriately sized balloon (balloon:scaffold diameter >1:1 and balloon diameter ≤scaffold diameter+0.5 mm).
STATISTICAL ANALYSIS
All analyses, except for the PSP analysis, were performed in the intent-to-treat (ITT) population according to randomised device assignment, regardless of treatment received. Comparison of treatment outcomes between the PSP and non-PSP subgroups was performed based on the “as-treated” population according to the type of stent actually deployed, and was restricted to patients treated with at least one BVS. In patients with multiple lesions, all lesions had to be treated per the PSP criteria to be in the PSP subgroup. Continuous variables are presented as mean±SD and were compared by Student’s t-tests. Binary variables are presented as counts and percentages and were compared by Pearson’s chi-square test when Cochran’s rule (expected cell size >5) was met; otherwise, Fisher’s exact test was used. Time-to-first-event curves were plotted using Kaplan-Meier (KM) estimates and were compared with the log-rank test. Independent predictors of three-year TLF were determined by multivariable logistic regression using stepwise selection, where variables were entered into the model at the 0.10 significance level and removed at the 0.05 level based on the Wald chi-square statistic. The following variables were entered into this model: current tobacco use, hypertension requiring medications, baseline RVD, calcification (moderate/severe) and randomisation arm. The Hosmer and Lemeshow goodness-of-fit test was performed to examine overall model fit. All statistical analyses were performed with SAS version 9.3 (SAS Institute, Cary, NC, USA).
Results
PATIENTS AND BASELINE CHARACTERISTICS
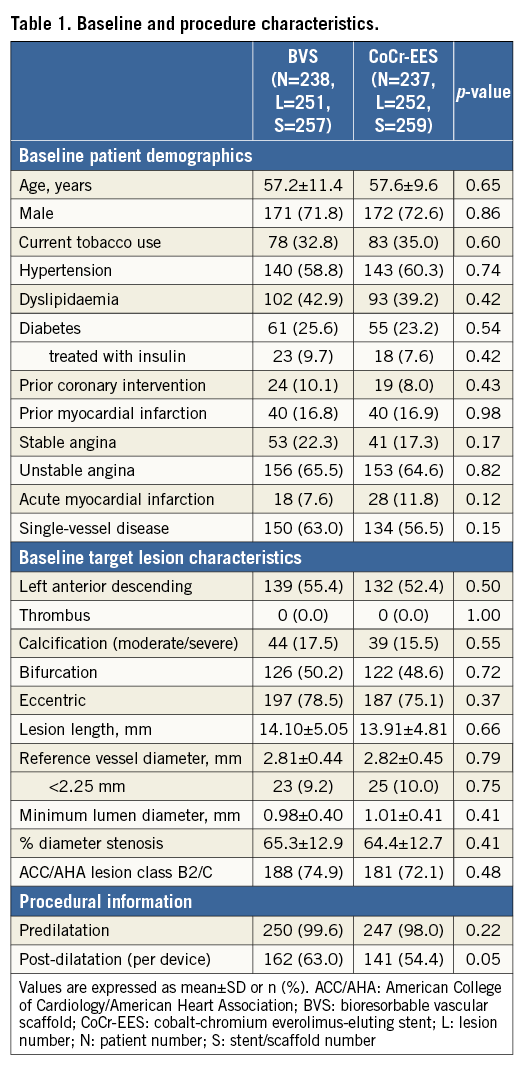
Between July 2013 and March 2014, 480 patients were randomly assigned to receive either BVS (N=241) or CoCr-EES (N=239). There were four withdrawals and one lost to follow-up in the BVS arm and two withdrawals and two lost to follow-up in the CoCr-EES arm prior to the three-year follow-up (Figure 1). In addition, five patients (three BVS and two CoCr-EES) withdrew from the study following randomisation but prior to any study device use; these patients were excluded from all data analyses. The clinical follow-up rates at one, two and three years were thus 98.8%, 98.1% and 98.1%, respectively, and were similar in the two randomised groups. Baseline and procedure characteristics were well balanced between the treatment arms except for post-dilatation, which was performed more commonly in the BVS group (Table 1). Of note, 48/501 target lesions (9.6%) were <2.25 mm by QCA, with similar distribution in the two treatment groups. The three-year usage rates of aspirin (91.1% vs. 88.6%, p=0.36), an ADP antagonist (25.2% vs. 23.6%, p=0.69), and dual antiplatelet therapy (DAPT) (20.8% vs. 18.2%, p=0.49) were similar in patients randomised to BVS vs. CoCr-EES, respectively.
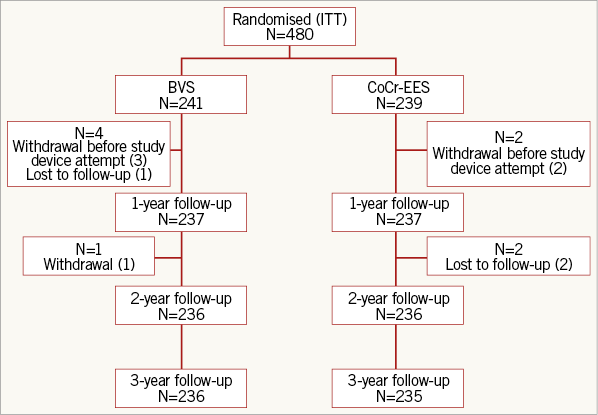
Figure 1. Patient disposition. BVS: bioresorbable vascular scaffold; CoCr-EES: cobalt-chromium everolimus-eluting stent; ITT: intent-to-treat
CLINICAL OUTCOMES UP TO THREE YEARS
The three-year rates of TLF (5.5% and 4.7%, p=0.68) and the PoCE (11.9% and 11.9%, p=0.99) were comparable in the BVS and CoCr-EES groups, respectively (Table 2, Figure 2). By multivariable logistic regression analysis, moderate or severe calcification and current smoking were significant independent predictors of three-year TLF (Figure 3). The adjusted three-year rates of TLF were similar with BVS compared to CoCr-EES (OR [95% CI]:1.17 [0.51, 2.69], p=0.71). There were also no significant differences between the treatment groups for any of the composite and component endpoints up to three years.
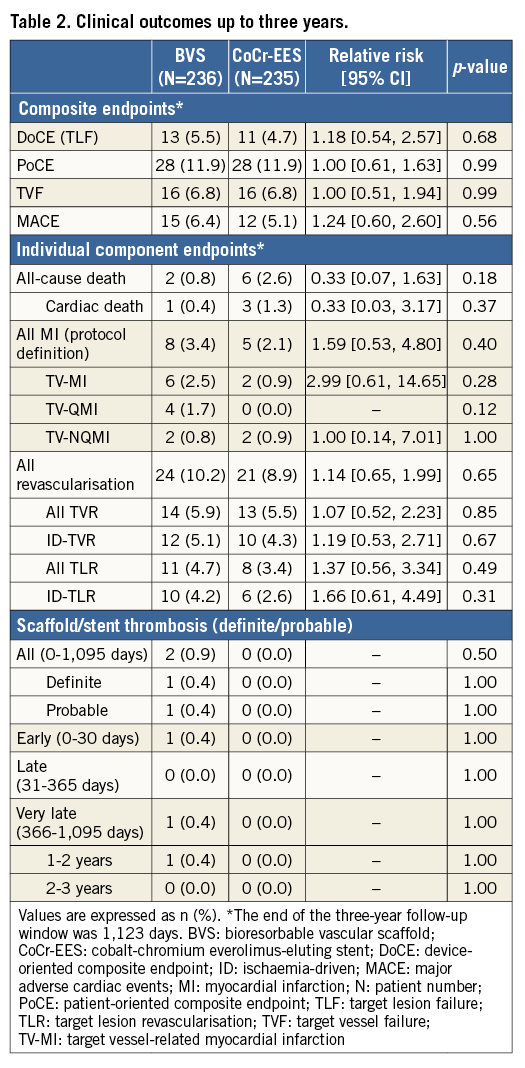
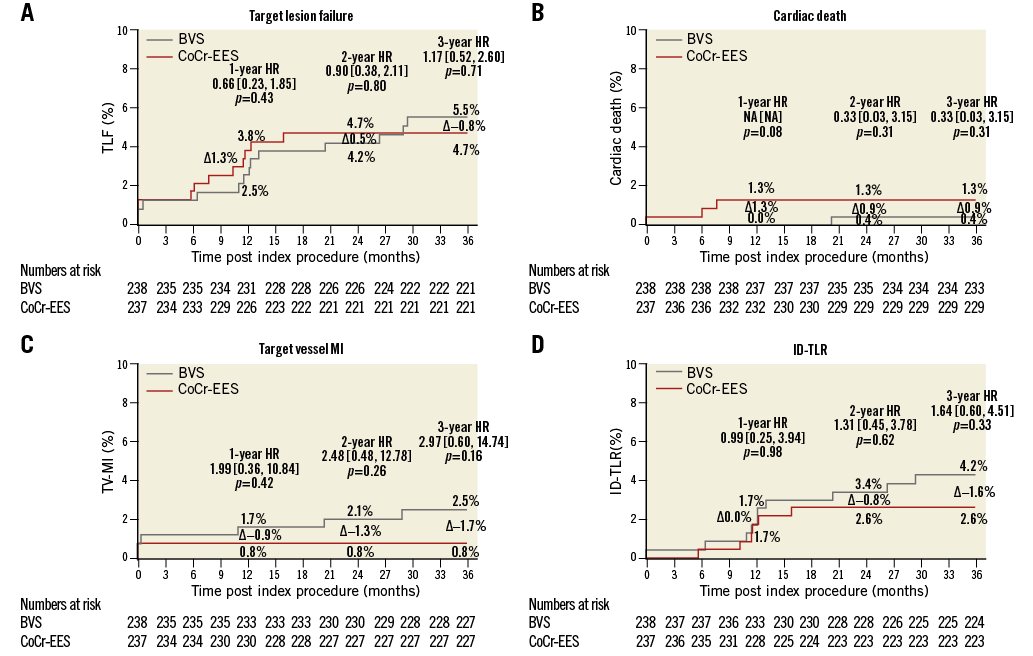
Figure 2. Time-to-event curves for TLF and its components in the intent-to-treat population. Time-to-event curves up to 1,095 days for target lesion failure (TLF) (A), cardiac death (B), target vessel-related myocardial infarction (TV-MI) (C), and ischaemia-driven target lesion revascularisation (ID-TLR) (D). HR: hazard ratio
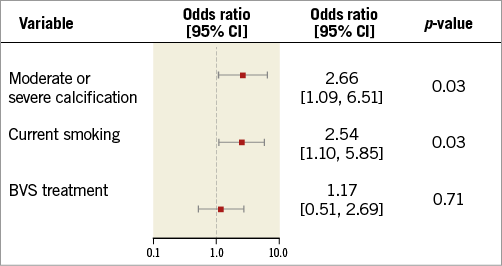
Figure 3. Multivariable logistic regression analysis of three-year TLF in the intent-to-treat population. CI: confidence interval; TLF: target lesion failure
STENT/SCAFFOLD THROMBOSIS
Definite or probable ST by three years occurred in two (0.9%) BVS patients and zero CoCr-EES patients (p=0.50). One BVS-treated patient developed a probable ST 15 days post procedure. This patient experienced chest pain and was diagnosed with ST-segment elevation myocardial infarction; angiography was not performed and the symptoms resolved with medical treatment. At baseline, the target lesion QCA RVD was 2.62 mm. However, a small BVS was implanted (2.5×18 mm), post-dilatation was not performed, and the final in-segment and in-stent minimal lumen diameters (MLDs) were 1.97 mm and 2.08 mm, respectively (diameter stenoses 24.8% and 20.7%, respectively), representing marked underexpansion. Only one definite ST occurred in a BVS-treated patient (at 622 days). This event occurred in a 3.0×18 mm BVS implanted in a lesion with a QCA RVD of 2.95 mm. Predilatation was performed, but no post-dilatation was carried out. By QCA, the post-procedure in-segment and in-stent MLDs were 2.29 mm and 2.42 mm, respectively (diameter stenoses 19.7% and 15.2%, respectively), again representing substantial scaffold underexpansion. Both patients were taking DAPT at the time of the ST event.
IMPACT OF VESSEL SIZE AND PROCEDURAL TECHNIQUE IN BVS-TREATED PATIENTS
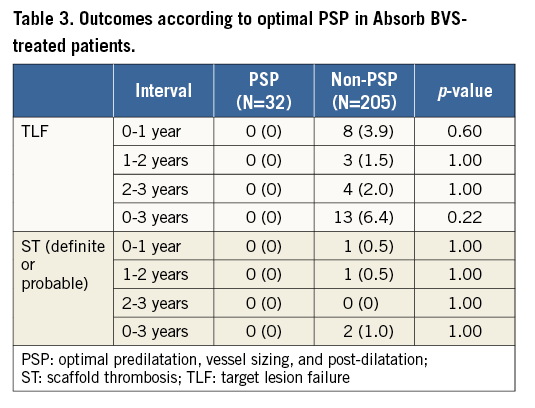
As pre-specified, all three components of the PSP criteria were met in 32/237 BVS-treated patients (13.5%); 236/237 (99.6%) met the optimal predilatation criterion, 194/237 (81.9%) met the optimal vessel size criterion, and 40/237 (16.9%) met the optimal post-dilatation criterion. None of the 32 BVS-treated patients in whom all three PSP criteria were met developed TLF or ST up to three years of follow-up. In contrast, 13/203 (6.4%) and 2/202 (1.0%) BVS-treated patients in whom one or more PSP criteria were not met developed TLF and ST, respectively, within three years (Table 3).
Discussion
The present study demonstrates that adverse events up to three years in the ABSORB China trial occurred with similar frequency in patients treated with BVS and CoCr-EES. The three-year point after BVS implantation is an important landmark as this is the time when preclinical studies have demonstrated that scaffold polymer absorption is complete18 (although in humans a small amount of residual polymer has occasionally been demonstrated to be present beyond that time point19). Clinical events may become less common after complete polymer bioresorption, as suggested in the ABSORB Cohort B study13. Moreover, whereas the two- and three-year rates among CoCr-EES-treated patients were similar in the ABSORB II, Japan and China trials14,15,20, the two- and three-year outcomes with BVS in ABSORB China were more favourable than in ABSORB II and Japan. While differences in the enrolled patient populations and study processes (or chance) cannot be excluded as explanations for this observation, differences in technique may also have contributed to the low rates of adverse events with BVS in ABSORB China.
Understanding of the lesion-related and technique factors that impact on BVS outcomes with the first-generation Absorb scaffold is evolving. In particular, implantation of BVS in very small vessels (RVD <2.25 mm by QCA) and suboptimal procedural techniques have been associated with increased adverse event rates with BVS16,21. In the present study, only two BVS-treated patients (0.9%) developed ST within three years, including only one very late ST (between one and three years), a lower rate than that observed in ABSORB II and ABSORB Japan. As intravascular imaging was not performed at the time of the very late ST event, whether malapposition or structural discontinuities of the scaffold contributed to its occurrence is unknown22. The fact that the very late ST event did not occur in a very small vessel in the present study is consistent with observations from the ABSORB II and ABSORB Japan trials14,15. Thus, implantation of BVS in very small vessels (QCA RVD <2.25 mm) appears to be a risk factor for scaffold thrombosis within one year, but not necessarily after one year. Of note, only 9.6% of the enrolled target lesions in ABSORB China had an RVD <2.25 mm by QCA, a smaller proportion than those treated in the ABSORB II (19.1%), ABSORB III (18.3%) and ABSORB Japan (14.4%) trials, which may have contributed to the low BVS ST rates in the present study.
In both ST cases in the present study BVS were underexpanded, and had a relatively small MLD. The current-generation BVS have a strut thickness of 157 µm, and achieving a reasonable MLD is especially important to minimise BVS ST. Puricel et al reported from a multicentre registry that the one-year risk of ST with BVS was increased with post-procedure MLD below 2.4 mm (for the 2.5 and 3.0 mm BVS) and below 2.8 mm (for the 3.5 mm BVS)16. Moreover, routine high-pressure non-compliant balloon post-dilatation may be of particular benefit in minimising very late scaffold thrombosis23. The relatively high rate of post-dilatation in the present study may have contributed to the low rate of very late BVS ST. Moreover, no cases of very late BVS ST between one and three years occurred in the ABSORB II, III, China, Japan and EXTEND studies when BVS implantation was avoided in very small vessels and optimal post-dilatation was performed.
Of note, both BVS ST events occurred in patients taking DAPT. Further study is required to determine the optimal duration of DAPT after BVS implantation. However, as prolonged DAPT is useful in reducing ST even after CoCr-EES24, it is likely that there will be a benefit of prolonged DAPT after BVS implantation in reducing ST and MI for at least three years, the time at which bioabsorption is complete. Such DAPT duration decisions should be made considering the likelihood of benefit vs. potential harm from increased bleeding, accounting for the individual risk profile of each patient. Finally, whether prolonged DAPT is relatively less beneficial if optimal BVS implantation technique is used deserves further study.
Moderate or severe lesion calcification and current smoking (but not device type) were identified as independent predictors of three-year TLF, both of which have been associated with reduced event-free survival in prior studies25,26. Moderate and severe calcification may result in underexpansion of both stents and scaffolds, and can pose challenges for optimal implantation of BVS27. The average rate of current tobacco use in the study was 33.9%, relatively high compared to the other ABSORB trials, reflecting the prevalence of smoking in China.
Limitations
Several limitations of the present study deserve mention. ABSORB China was an open-label trial, and some degree of bias cannot be excluded. Nevertheless, the effect of potential bias on outcomes was minimised by use of an independent clinical events committee to adjudicate outcome measures, and an independent angiographic core laboratory to analyse angiograms. The patients and lesions enrolled were relatively non-complex, and by protocol heavily calcified lesions, true bifurcation and left main lesions, and chronic total occlusions were excluded. The extent to which event rates may have been increased in more complex lesions is unknown. Analysis of the relationships between procedural technique and outcomes was post hoc, was non-randomised and not adjusted for differing patient or lesion characteristics, and should be considered hypothesis-generating. The present study was underpowered to determine the influence of PSP on outcomes. Additional analyses from the pooled ABSORB clinical trial program are underway to assess the extent to which optimal technique impacts on outcomes after BVS. Finally, the sample size was modest, was not powered for clinical endpoints, and events between one and three years were particularly infrequent. Larger studies with longer-term follow-up are required to generate more precise estimates of early, late and very late event rates after BVS, and to establish optimal techniques for BVS implantation more firmly.
Conclusions
With follow-up up to three years in the multicentre, randomised ABSORB China trial, BVS had similar safety and effectiveness outcomes compared to CoCr-EES. Outcomes with the relatively thick-strut first-generation BVS may be further improved by avoidance of device implantation in very small vessels, and by otherwise optimising implantation technique, including avoiding device-vessel size mismatch and routine post-dilatation with high-pressure non-compliant balloons.
| Impact on daily practice With follow-up up to three years in ABSORB China, BVS continued to demonstrate results similar to CoCr-EES (TLF 5.5% vs. 4.7%, p=0.68; definite/probable ST 0.9% vs. 0.0%, p=0.50). Avoiding BVS implantation in very small vessels and optimising procedural technique may further improve BVS outcomes. Long-term follow-up in large-scale studies is required to provide accurate estimates for the differences in outcomes between BVS and CoCr-EES, and to determine the extent to which any differences can be mitigated by optimal technique. |
Acknowledgements
We thank all the investigators who participated and contributed towards the enrolment and follow-up of the ABSORB China study.
Funding
The ABSORB China trial has been sponsored and funded by Abbott Vascular.
Conflict of interest statement
R. Gao has received a research grant from Abbott Vascular. Y. Han has received an institutional research grant from Lepu Medical. H.C. Kuo, S.W. Ying, W.F. Cheong, Y. Zhang, and X. Su are employees of Abbott Vascular. G.W. Stone is the global chairman for the ABSORB clinical trial program (uncompensated), and a consultant to Reva Corp. The other authors have no conflicts of interest to declare.

