Abstract
Aims: Clinical data on the early and midterm outcomes of bioresorbable vascular scaffolds (BVS) in routine clinical practice are limited. To fill this gap, we report on the early and midterm clinical outcomes of PCI with everolimus-eluting BVS from the large multicentre GHOST-EU registry.
Methods and results: Between November 2011 and January 2014, 1,189 patients underwent percutaneous coronary intervention with one or more BVS (Absorb BVS; Abbott Vascular, Santa Clara, CA, USA) at 10 European centres. The primary outcome of interest was target lesion failure (TLF), defined as the combination of cardiac death, target vessel myocardial infarction, or clinically driven target lesion revascularisation (TLR). A total of 1,731 Absorb BVS were implanted at a mean of 12.3±3.4 atm. Technical success was achieved in 99.7% of cases. TLF was recorded in 67 of 1,189 patients at a median of 109 (interquartile range 8-227) days after implantation. The cumulative incidence of TLF was 2.2% at 30 days and 4.4% at six months. The annualised rate of TLF was 10.1%. At six months, the rate of cardiac death was 1.0%, target vessel myocardial infarction was 2.0%, TLR was 2.5%, and target vessel revascularisation was 4.0%. Diabetes mellitus was the only independent predictor of TLF (hazard ratio 2.41, 95% confidence interval: 1.28-4.53; p=0.006). The cumulative incidence of definite/probable scaffold thrombosis was 1.5% at 30 days and 2.1% at six months, with 16 of 23 cases occurring within 30 days.
Conclusions: “Real-world” outcomes of BVS showed acceptable rates of TLF at six months, although the rates of early and midterm scaffold thrombosis, mostly clustered within 30 days, were not negligible.
Introduction
Current-generation drug-eluting stents (DES), with thinner struts and release of limus family analogues from durable or biodegradable polymers, hold several advantages over previous-generation DES and bare metal stents, including reduced rates of restenosis, stent thrombosis and need for repeat revascularisation1,2. However, permanent caging of the vessel with metal is still perceived as a limitation of DES, in that it prevents late luminal enlargement, adaptive shear stress and late expansive remodelling.
Bioresorbable vascular scaffolds (BVS) promise to address some of the pending issues with current-generation DES. With BVS, transient scaffolding of the vessel prevents acute closure and recoil, drug elution counteracts neointimal proliferation, and complete bioresorption after two to four years, together with vessel lumen enlargement, plaque/media reduction and vasomotion restoration, finally realises the paradigm of vascular restoration therapy3. Theoretically, because drug elution and scaffolding are temporary, BVS eliminate some of the traditional triggers for very late stent thrombosis, including non-endothelialised struts and polymers. Additional putative benefits include no restrictions on future percutaneous or surgical procedures, no artefacts on non-invasive imaging techniques due to the partial volume averaging associated with metallic stents, and the elimination of the concern that some patients have about a foreign material remaining in their coronary arteries lifelong3.
Although all these concepts are attractive, the outcomes of BVS remain to be determined with respect to safety and efficacy in routine clinical practice. Early clinical testing has been limited by patient selection4, follow-up restriction to 30 days5 or the small sample size, the latter precluding a clear understanding of the true magnitude of low-frequency events (i.e., scaffold thrombosis). Few reports from small series have focused on the outcomes of BVS in specific patient populations, such as patients with diabetes6 or those presenting with acute coronary syndromes7 or ST-segment elevation myocardial infarction8,9. Yet, there is a paucity of data regarding the use of BVS in “real-world” patients undergoing percutaneous coronary interventions (PCI). To fill this gap, we report on the early and midterm clinical outcomes of PCI with everolimus-eluting BVS from a large multicentre registry.
Methods
STUDY DESIGN AND PATIENT POPULATION
The GHOST-EU (Gauging coronary Healing with biOresorbable Scaffolding plaTforms in EUrope) registry is a retrospective non-randomised, multicentre registry conducted at 10 European centres in Germany, Italy, Poland and the United Kingdom. The registry includes 1,189 patients undergoing single or multivessel percutaneous coronary intervention with the current generation of the everolimus-eluting BVS device (Absorb BVS; Abbott Vascular, Santa Clara, CA, USA) between November 11, 2011, and January 29, 2014. The study was designed during two conferences among the investigators. The sponsor (Abbott Vascular) financed only the conferences and had no role in data analysis, drafting or editing of the final manuscript. Due to the nature of the study the patients were not retrospectively requested to provide consent, but local institutional ethical committees approved the use of aggregated clinical data on the condition that the subjects’ identities were concealed. Baseline clinical and angiographic variables, procedural characteristics, and outcomes were identified, and a worksheet with all variables of interest was supplied to the study sites. Data from all sites were then entered into a central database, held in Catania, Italy, which generated the analysis presented in this manuscript. Source verification, quality control, and queries generation from the coordinating centre to the participating sites were undertaken to account partly for the unavoidable bias of site-reported data collection and adjudication.
All patients with coronary artery lesions suitable for stenting were eligible for recruitment to the registry. Patients for whom implantation with an Absorb BVS was intended were included. The registry population encompassed a relatively large number of patients with clinical and angiographic characteristics that did not fit the entry and exit criteria of the ongoing ABSORB II trial10, reflecting a broader “real-world” use. This included patients with a baseline acute myocardial infarction (MI), moderate and severe chronic kidney failure, poor ventricular function, bifurcated and ostial lesions, in-stent restenosis, chronic total occlusions and left main disease. Patients were permitted to have one or more Absorb BVS implanted, and concurrent implantation of DES or bare metal stents was allowed at the operator’s discretion.
Objectives
The primary objective of the GHOST-EU registry was to evaluate the safety and overall clinical performance of the Absorb BVS in real-world patients. The secondary objective was to collect a large number of patients to enable meaningful exploratory analyses in clinical and angiographic subsets of interest, which will be reported separately.
Procedures and follow-up
All interventions were performed according to current standards of PCI, with mandatory predilatation and scaffold implantation at a pressure not exceeding the burst pressure rate. The decision to choose a specific treatment strategy, including post-dilatation, was left to the operator’s discretion. A loading dose of aspirin 250-500 mg was given before PCI, unless patients were already on chronic aspirin therapy, followed by 75-100 mg oral daily indefinitely thereafter. A loading dose of clopidogrel (600 mg), prasugrel (60 mg), or ticagrelor (180 mg) was administered before or immediately after PCI, unless patients were already on chronic maintenance therapy, followed by a maintenance dose of clopidogrel (75 mg od), prasugrel (10 mg od), or ticagrelor (90 mg bid) for six to 12 months. The use of glycoprotein IIb/IIIa inhibitors was at the physician’s discretion.
Cardiac enzymes were measured after the procedure according to local hospital practice, including measurement of creatine kinase, creatine kinase-myocardial band, and troponin. Clinical follow-up was obtained by clinical visit and/or through telephone contact, according to a schedule specific for each site, for the occurrence of major adverse cardiac events, including all-cause death, MI and repeat revascularisation. Referring cardiologists, general practitioners and patients were contacted whenever necessary for further information. There was no independent or external monitoring of data entry.
Device description
The Absorb BVS is made of semi-crystalline poly-L-lactide (PLLA), coated (strut thickness of 155 μm, strut width of 188-213 μm depending on size) with a poly-D, L-lactide that controls the release of everolimus (100 μg/cm2 of scaffold). Both PLLA (backbone) and poly-D, L-lactide (coating) are fully bioresorbable and degrade to lactic acid in about two to four years.
Outcomes and definitions
The primary outcome of interest was a device-oriented composite endpoint (target lesion failure [TLF]), defined as the combination of cardiac death, target vessel MI, or clinically driven target lesion revascularisation (TLR), either percutaneous or surgical11. Secondary outcomes of interest included the components of TLF, target vessel failure (TVF), defined as the composite of cardiac death, target vessel MI, or clinically driven target vessel revascularisation (TVR), and scaffold thrombosis (ST). Deaths that could not be attributed to another cause were regarded as cardiac deaths. Recurrent MI was defined according to the universal definition12. ST was classified according to the Academic Research Consortium criteria11.
Statistical analysis
Continuous variables are presented as mean±standard deviation or median (interquartile range [IQR]) and were compared using a Student’s unpaired t-test for comparisons. Categorical variables are presented as counts and percentages, and were compared using chi-square or Fisher’s exact tests, as appropriate. Kaplan-Meier methods were used to derive the event rates at follow-up and to plot time-to-event curves. Patients not eligible for six-month follow-up were considered at risk until the date of last contact, at which point they were censored. The incidence of TLF and other secondary outcomes was provided as cumulative incidence and annualised rates. The annualised rate was defined as the number of patients who had the event divided by the total number of patient-years, and it was expressed as a number per 100 patient-years of observation.
A Cox proportional hazards analysis was used to identify the independent predictors of TLF. First, the following covariates were screened in univariate models: 1) clinical variables (age, sex, current smoking, diabetes mellitus, previous PCI, previous coronary artery bypass grafting, acute coronary syndrome at presentation, renal insufficiency, i.e., estimated glomerular filtration rate <60 mL/min); 2) angiographic variables (treatment of in-stent restenosis, ostial lesion, chronic total occlusion, bifurcation lesion, thrombus-containing lesion, lesion type class B2 or C according to the American College of Cardiology [ACC]/American Heart Association [AHA] classification); 3) procedural variables (average scaffold diameter, total scaffold length, average number of scaffolds, concurrent implantation of metallic stents, intravascular ultrasound use, optical coherence tomography use, post-dilation, use of prasugrel or ticagrelor). Lesion-level variables were summarised as worst case per patient if multiple lesions were present. Second, a multivariable analysis of predictors selected at p<0.20 by univariate analysis was performed to identify independent predictors of TLF and to estimate adjusted hazard ratios (HRs) and 95% confidence intervals (CIs). To avoid overfitting data and to select the best predictors, a bootstrap method was used, in which a logistic regression analysis was repeated for each of 1,000 bootstrap samples, and finally only predictors selected in at least 95% of the samples were selected. Addition of further variables and interaction terms proved unnecessary. The validity of the proportionality assumption was verified for all covariates by a visual examination of the log (minus log) curves and a test based on the Schoenfeld’s residuals.
All probability values reported are two-sided, and a probability value <0.05 was considered significant. All data were processed using the Statistical Package for Social Sciences, version 18 (SPSS Inc., Chicago, IL, USA).
Results
PATIENT POPULATION
Between November 11, 2011, and January 29, 2014, 1,189 patients underwent PCI with one or more Absorb BVS. Of these, 18.4% of patients received both Absorb BVS and conventional metallic stents. As of April 7, 2014, clinical follow-up was available at a median of 189 (IQR 120-284) days, with follow-up at 30 days and six months available in 94% and 76% of patients, respectively. Table 1 and Table 2 summarise the baseline demographics, clinical and lesion characteristics of the overall study population. The mean age was 62.2±11.0 years, with male patients comprising 79.4% of the total population. Diabetes mellitus was present in 24.8% (8.9% were on insulin therapy). Multivessel disease was present in 34.8% of patients. The mean reference vessel diameter and lesion length were 3.0±0.5 and 19.4±14.4 mm, respectively. A total of 51% of lesions were of class B2 or C according to the ACC/AHA classification.
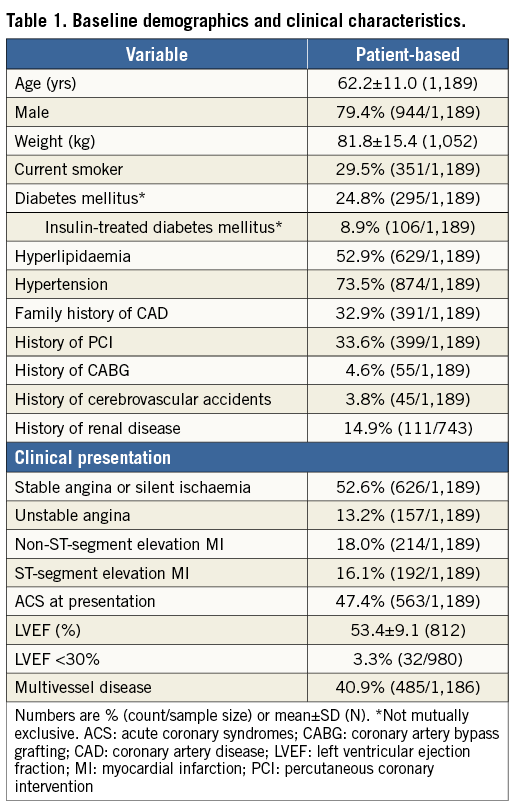
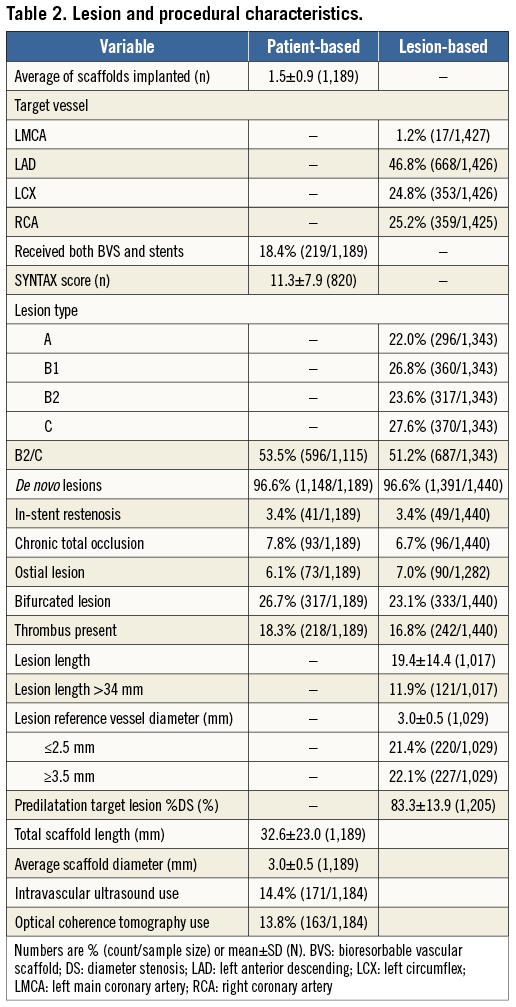
PROCEDURAL DETAILS AND MEDICATIONS
A total of 1,731 Absorb BVS were implanted at a mean of 12.3±3.4 atm. Predilation was performed in 1,405/1,440 (98%) of lesions. The mean number of treated lesions per patient was 1.2±0.5. Mean scaffold length per lesion was 28.1±18 mm and placement of overlapping scaffolds was required in 17.3% of lesions. Post-dilation was performed in 712/1,440 (49%) of lesions. The final Thrombolysis In Myocardial Infarction flow was 3 in 98.4% of cases. Technical success, defined as visually estimated residual in-scaffold diameter stenosis <30%, was achieved in 99.7% of cases. Dual antiplatelet therapy was prescribed at discharge in all patients and recommended for at least 12 months in 93.6% of them. Clopidogrel, prasugrel and ticagrelor were prescribed in 73.2%, 26.2% and 0.6% of patients, respectively. At six months, dual antiplatelet discontinuation was documented in 1.2% of patients.
CLINICAL OUTCOMES
TLF was recorded in 67 of 1,189 patients at a median of 109 (IQR 8-227) days after Absorb BVS implantation. TLF occurred between 0 and 30 days in 25 of 67 (37%) patients, between 30 and 180 days in 20 of 67 (30%) patients, and after 180 days in 22 of 67 (33%) patients. The cumulative incidence of TLF according to the Kaplan-Meier method was 2.2% at 30 days and 4.4% at six months (Table 3, Figure 1). The annualised rate of TLF was 10.1%. At six months, the rate of cardiac death was 1.0%, target vessel MI was 2.0%, TLR was 2.5%, TVR was 4.0% and TVF was 4.9% (Table 3, Figure 1). The corresponding annualised rates for cardiac death, target vessel MI, TLR, TVR and TVF were 1.6%, 3.6%, 7.0%, 10.2% and 11.9%, respectively. Among patients receiving Absorb BVS plus metallic stents, the six-month TLF rate was 5.6%.
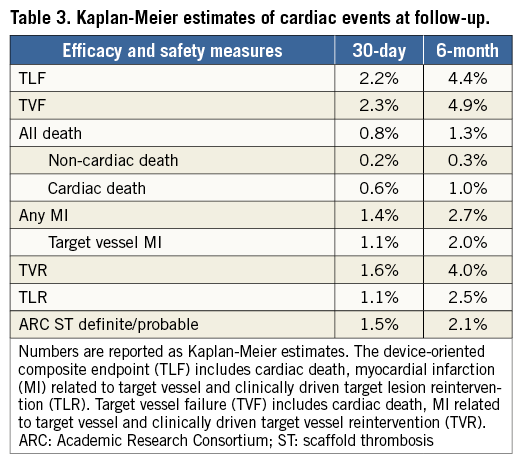
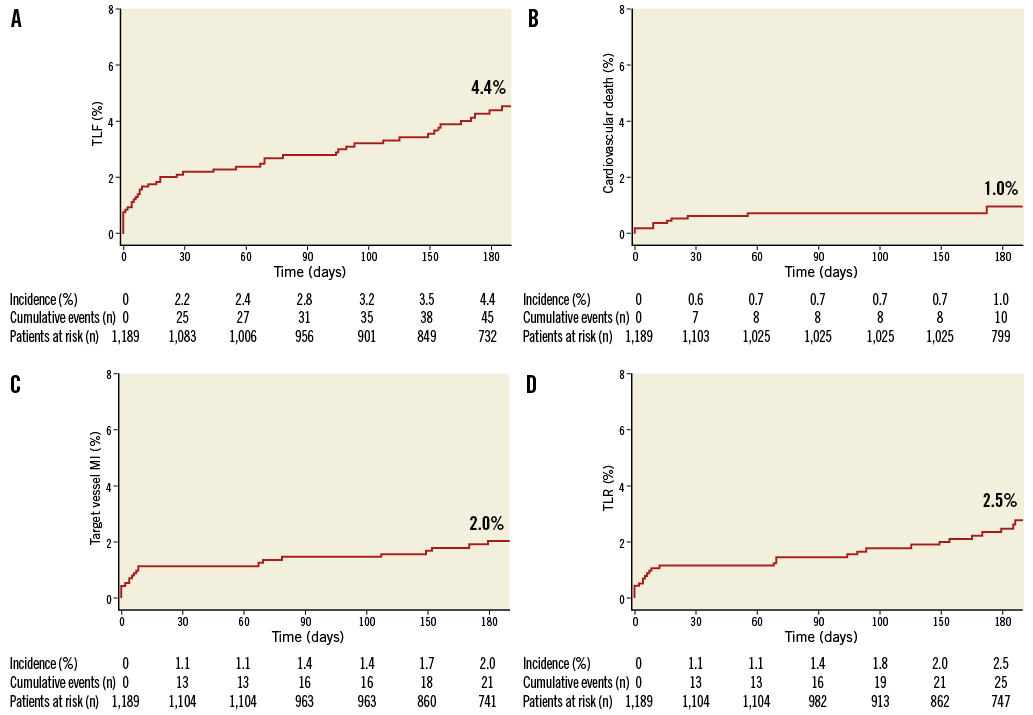
Figure 1. Kaplan-Meier curves showing A) target lesion failure (TLF), B) cardiovascular death, C) myocardial infarction (MI), and D) target lesion revascularisation (TLR).
PREDICTORS OF TARGET LESION FAILURE
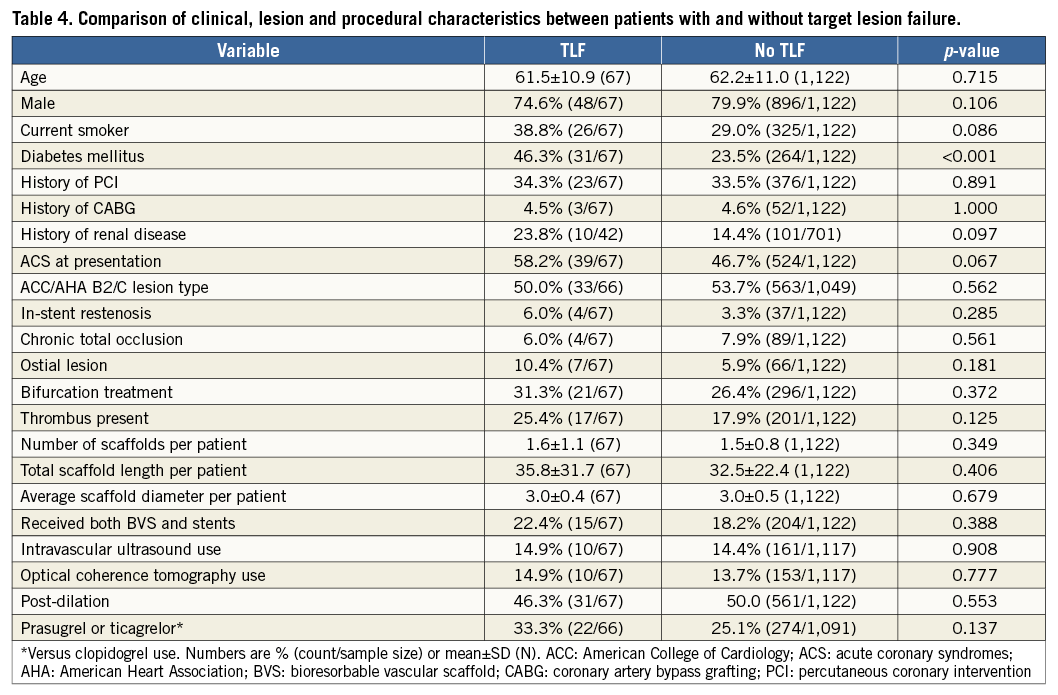
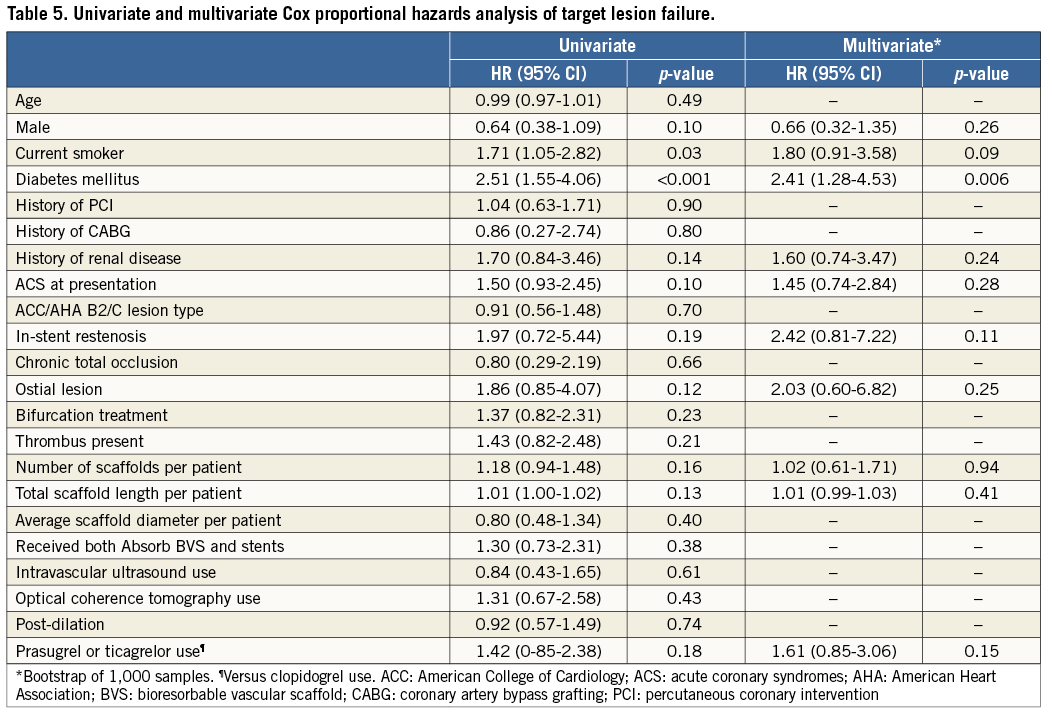
Patients with TLF and those without generally had similar clinical, lesion and procedural characteristics, but those with TLF presented more commonly with diabetes mellitus (Table 4). The results of the Cox proportional analysis are shown in Table 5. Diabetes mellitus was the only independent predictor of TLF (HR 2.41, 95% CI: 1.28-4.53; p=0.006). Other clinical, lesion and procedural variables did not seem to be independently associated with an increased risk of TLF within the follow-up period.
LEARNING CURVE
To investigate the impact of the learning curve on clinical outcomes, patients were categorised by centre experience with the Absorb BVS at the time of PCI (arbitrarily defined as >50 patients already treated with the Absorb BVS). Compared with patients treated within the first 50 cases, those treated after 50 cases more commonly presented with an ACS (54.0% vs. 37.7%, p<0.001) and were treated more frequently for ostial and thrombotic lesions. Intravascular imaging and post-dilation were performed more frequently at the beginning of the learning curve. At six months, TLF occurred in 3.2% of patients treated within the first 50 cases, and 5.2% of patients treated after 50 cases. The adjusted HR for the risk of TLF by BVS experience was 2.29 (95% CI: 1.13-4.64, p=0.02). Diabetes mellitus remained significantly associated with TLF when centre experience with BVS was forced into the multivariable model (HR 2.38, 95% CI: 1.27-4.46, p=0.007).
SCAFFOLD THROMBOSIS
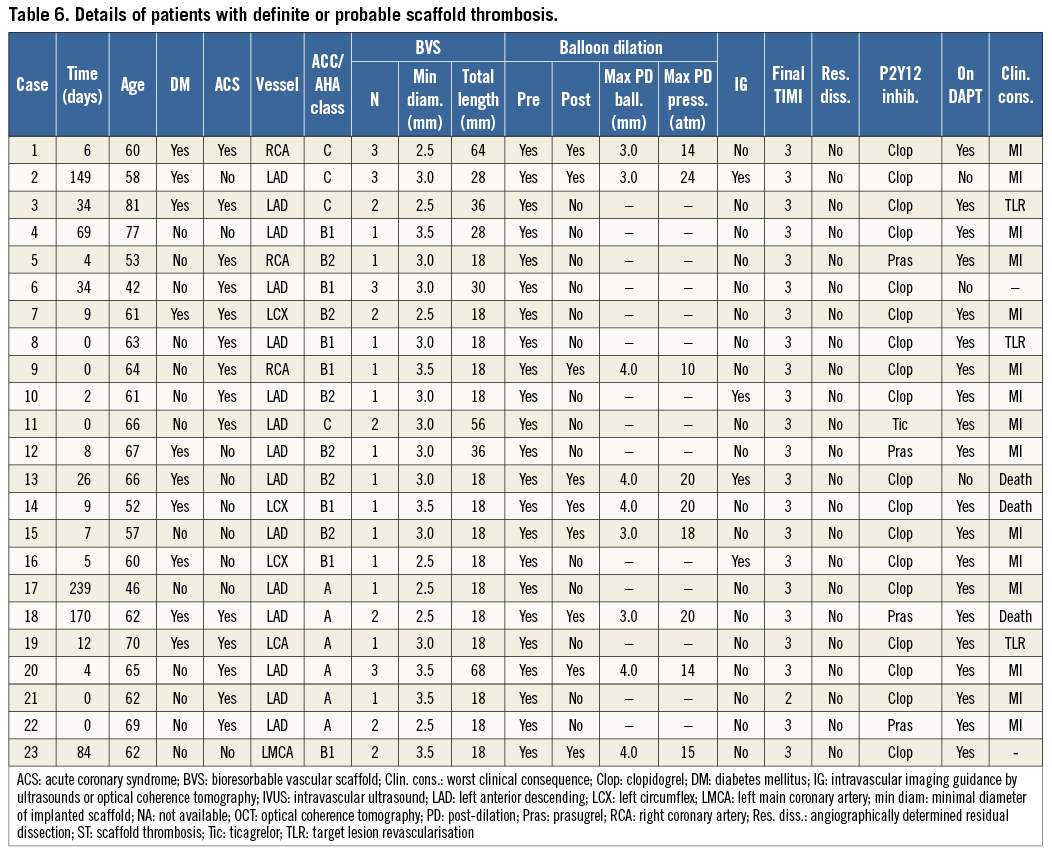
Definite ST was recorded in 20 of 1,189 patients at a median of 6.5 days (IQR 1.5-34) after Absorb BVS implantation. Three additional cases were labelled as probable ST according to the ARC definitions. Twenty of 23 patients (87%) were on dual antiplatelet therapy at the time of ST, and 14 (61%) presented with an acute coronary syndrome (four of them had an ST-elevation myocardial infarction) (Table 6). The annualised rate of definite/probable ST was 3.4%. ST occurred acutely (within 24 hours) in five of 23 (22%) patients, subacutely (after 24 hours but within one month after implantation) in 11 of 23 (48%) patients, and late (after 30 days) in seven of 23 (30%) patients. The median time to occurrence of early (acute or subacute) ST was five days (IQR 0-9). The median time to occurrence of late ST was 84 days (IQR 51.5-159.5). The cumulative incidence curve of definite/probable ARC-defined ST showed an initial steep rise with about 70% of cases occurring within 30 days, followed by a gentler trend up to six months (Figure 2). Overall, the cumulative incidence of ST was 1.5% at 30 days and 2.1% at six months. ST resulted in cardiac death in three of 23 patients (13%) and non-fatal reinfarction in 15 of 23 (65%) cases (Table 6). In patients treated within the first 50 cases and after 50 cases at each centre, the cumulative incidences of acute definite or probable ST were 0.7% and 2.0%, respectively.
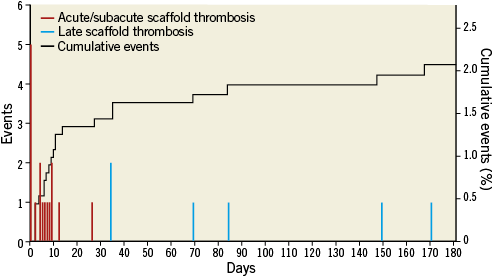
Figure 2. Distribution and cumulative incidence of scaffold thrombosis up to six months.
Discussion
We reported on clinical outcomes of patients enrolled in the largest contemporary registry of the Absorb BVS. Our results, encompassing data from 10 high-volume European centres, provide a meaningful real-world assessment of the early and midterm safety and efficacy of the Absorb BVS in routine clinical practice. Overall, Absorb BVS were associated with acceptable rates of target lesion failure at 30 days and six months, particularly when considering the complexity of the patients and lesions treated. However, signals of scaffold thrombosis in 1.5% of patients at 30 days and 2.1% at six months were noted that exceed the incidences typically reported in contemporary all-comers registries and trials of second-generation DES2,13.
In the GHOST-EU registry, complete outcomes data were available in 94% of patients at 30 days and 76% at six months. This latter percentage is explained by the notion that a proportion of patients were not eligible for six-month follow-up at the time of the present analysis. To account for this limitation, data were reported both as Kaplan-Meier estimates, with censoring at six months, and annualised incidence densities (i.e., rates per 100 patient-years of observation). Notably, the TLF rates for patients who were eligible for six-month follow-up (4.4%) and those with complete six-month follow-up (5.1%) were similar to the 4.4% rate reported for the overall population. Since the mechanical support of the Absorb BVS decreases during bioresorption, the period where restenosis peaks with these novel platforms is unknown and may differ from metallic stents. Therefore, six months may be an insufficient time frame to capture the full efficacy of the device, hence longer follow-up is needed. Indeed, in the small Cohort B from the first-in-man ABSORB trial, cases of restenosis were identified both at early and late term14. While follow-up collection of the GHOST-EU registry is ongoing, the annualised TLF (10.1%) and TVF (11.9%) rates appear comparable to those reported for the second-generation zotarolimus- and everolimus-eluting DES in the RESOLUTE All-Comers and TWENTE trials15,16.
It is important to emphasise that the GHOST-EU registry includes patients treated in the participating centres who received at least one Absorb BVS at the operator’s discretion. As for any previous generation of stent platforms, operators are gaining experience with the new device in increasingly complex clinical and angiographic scenarios. As a result, patients included in this study presented with characteristics that represent exclusion criteria for enrolment in the ongoing ABSORB II10 and ABSORB III (ClinicalTrials.gov identifier NCT01751906) trials. Accordingly, the registry reflects the high-risk features of an unselected population rather than the poorly generalisable setting of a trial, with 25% of patients presenting with diabetes, 41% with multivessel disease, 47% with an acute coronary syndrome, 53% with an ACC/AHA B2/C lesion, and 27% undergoing PCI of at least one bifurcation lesion.
We were not able to identify significant predictors of TLF at six months, with the notable exception of diabetes. In our study, patients with diabetes carried a 2.4-fold increased risk of TLF compared to those without. This is consistent with the notion that diabetic patients undergoing PCI are exposed to a significantly increased risk of restenosis and reinfarction17. In a recent pooled analysis of the ABSORB Cohort B and ABSORB EXTEND trials, the cumulative incidences of TLF did not differ at one year between patients with (N=136) and without diabetes (N=415)6. However, the small number of patients with diabetes and the partly selected nature of the population included in that study may have accounted for the observed lack of difference. Indeed, the differential effect of diabetes is dependent on the case mix of the PCI population considered, being more pronounced in patients with complex lesions such as those included in our registry18.
The cumulative incidence of scaffold thrombosis was larger than anticipated and, to the best of our knowledge, the present study represents the first one reporting a non-negligible rate of this outcome with the Absorb BVS. We observed 20 cases of angiographically confirmed ST and three probable ST. Seventy percent of the cases occurred in the first month after PCI, at a median of five days, and most cases resulted in death or recurrent myocardial infarction. Mechanisms of stent thrombosis are known to differ at variable time intervals after PCI, with early events mostly attributable to procedural issues (i.e., dissection, incomplete stent apposition, incomplete stent expansion) and late events more likely linked to stent factors and vascular response19,20. In such instances of varied underlying pathology, whether dual antiplatelet therapy might be sufficient to suppress thrombosis is hypothetical but not supported by our data (i.e., 20 of 23 patients were on dual antiplatelet therapy at the time of ST). Conversely, clustering of ST within the early period suggests the need for scrupulous lesion selection and PCI techniques when using BVS, and the opportunity for systematic post-implantation assessment (i.e., intravascular imaging may result in additional post-dilation, which was performed in only nine of 23 patients who experienced ST). The principal aim of a large post-marketing multicentre registry is to assess the incidence of infrequent outcomes, including thrombosis, within the longest follow-up period. Based on this study, the rates of ST with the Absorb BVS resemble those of first-generation DES and apparently do not compare favourably with the perception of very low rates of thrombosis with second-generation DES in clinical trials1. For example, the incidences of definite or probable scaffold thrombosis at 30 days with zotarolimus- and everolimus-eluting stents were 0.8% and 0.4% in the large E-FIVE and XIENCE V USA registries, respectively, versus 1.5% in the present report21,22. However, the incidence of known predictors of thrombosis (i.e., bifurcation lesions, acute coronary syndromes, long lesions) was relatively high in the GHOST-EU database. Whether this signal corresponds to a true statistical difference can only be addressed by a randomised trial. Although any indirect comparison with first- and second-generation DES is invalidated by the non-randomised and single-arm nature of our registry and the differences in the complexity of the population and the lesions treated in different registries, the association between strut thickness and the risk of thrombosis has been previously suggested in ex vivo experimental models, with thick-strutted platforms (i.e., Absorb BVS, first-generation DES, thick-strut bare metal stents) described as being 1.5-fold more thrombogenic than thin-strutted counterparts (i.e., second-generation DES or even thin-strut bare metal stents)23. Whether the apparent early detrimental effect of strut thickness is counterbalanced by later benefits occurring when the scaffold is biodegraded needs to be clarified by longer follow-up and randomised comparisons versus best-in-class DES.
We were not able to demonstrate a significant effect of the learning curve on clinical outcomes of patients treated with the Absorb BVS. Patients treated after the first 50 cases more frequently presented with extended clinical (i.e., acute coronary syndromes) and angiographic (i.e., ostial and thrombotic lesions) characteristics compared with those treated in the early experience, and less frequently underwent intravascular imaging and post-dilation during the index procedure. We speculate that this may reflect the increasing confidence from the operators in using the Absorb BVS in more complex patient and clinical scenarios. However, patient- and lesion-specific variables that were different between the two levels of experience failed to emerge as significant predictors in the general multivariable model, which may be explained by the presence of unidentified interactions or residual confounders. As such, the observation that TLF and ST rates were numerically higher when more experience was accumulated raises a note of caution on the unselected use of BVS in all-comers PCI, and demands a meticulous assessment of patient and lesion eligibility before implantation, at least until mechanisms of TLF with BVS are better elucidated.
Limitations
Our study suffers from the obvious limitations of a single-arm, real-world multicentre registry where follow-up was not standardised, data were site-reported and clinical events were not adjudicated by an independent committee. There were no central angiographic and imaging core labs. Patients who received metallic stents together with BVS represented about 18% of the overall population, with no differences between those who experienced a TLF and those who did not. To account for the potential influence of these patients on the study outcomes, we applied TLF as the primary outcome of interest in keeping with ARC standards that require reporting of device-oriented composite endpoints to represent the efficiency and efficacy of a new device11. Another caveat is the lack of standardised selection criteria and operating protocols for BVS implantation. A systematic analysis of the outcome of different implantation techniques (i.e., use of intravascular imaging, post-dilation, use of compliant versus non-compliant balloons) is warranted.
Conclusions
Early and midterm results from the GHOST-EU registry suggest that “real-world” outcomes among 1,189 patients with relatively unselected clinical characteristics and lesions are acceptable and comparable to those reported in the literature for second-generation DES, with one-digit rates of target lesion and vessel failure at six months and low incidences of cardiac death and reinfarction. On the other hand, the scaffold thrombosis rate resembles that of first-generation DES, suggesting a negative impact of high strut thickness on this event.
| Impact on daily practice Several studies are ongoing to address the safety and efficacy of BVS in patients undergoing PCI. However, BVS have been approved in Europe since 2011, and many centres are increasingly using them for a variety of clinical and angiographic presentations, despite the paucity of compelling comparative data. In the GHOST-EU registry, the use of BVS in relatively unselected patients and lesions was shown to be associated with excellent feasibility and favourable outcomes at both early and mid term. However, clustering of thrombotic events mostly in the first 30 days after implantation suggests some room for improvement in terms of lesion selection and optimisation of the implantation. While long-term data are awaited, studies are also warranted to explore the role of procedural factors in determining the early outcomes of BVS. |
Conflict of interest statement
D. Capodanno has received payments as an individual for: a) consulting fees from Eli-Lilly/Daiichi Sankyo and AstraZeneca; b) speaker’s honoraria from Stentys and Eli-Lilly/Daiichi Sankyo. T. Gori has received payments as an individual for speaker’s honoraria from Abbott Vascular. H. Nef has received payments as an individual for: a) research grants from Abbott Vascular; b) speaker’s honoraria from Abbott Vascular and Elixir Medical. A. Latib has received payments as an individual for: a) consulting fees from Medtronic; b) research grant from Angioscore. J. Mehilli has received payments as an individual for speaker’s honoraria from Abbott Vascular, Terumo, Biotronik, Eli-Lilly/Daiichi Sankyo. M. Lesiak has received payments as an individual for advisory board and speaker’s honoraria from Abbott Vascular, AstraZeneca, B Braun, Biotronik and Boston Scientific. C. Di Mario has received institutional payments for a research grant from Abbott Vascular. T. Münzel has received payments as an individual for speaker’s honoraria from Abbott Vascular. C. Tamburino has received institutional payments for a research grant from Edwards Lifesciences and payments as an individual for speaker’s honoraria from Abbott Vascular, Medtronic and Edwards Lifesciences. None of the other authors has any conflicts of interest to declare.

